Prelamin A and lamin A appear to be dispensable in the nuclear lamina
- PMID: 16511604
- PMCID: PMC1386109
- DOI: 10.1172/JCI27125
Prelamin A and lamin A appear to be dispensable in the nuclear lamina
Abstract
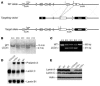
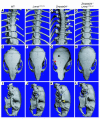
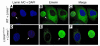
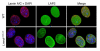
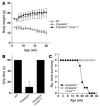
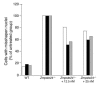
Lamin A and lamin C, both products of Lmna, are key components of the nuclear lamina. In the mouse, a deficiency in both lamin A and lamin C leads to slow growth, muscle weakness, and death by 6 weeks of age. Fibroblasts deficient in lamins A and C contain misshapen and structurally weakened nuclei, and emerin is mislocalized away from the nuclear envelope. The physiologic rationale for the existence of the 2 different Lmna products lamin A and lamin C is unclear, although several reports have suggested that lamin A may have particularly important functions, for example in the targeting of emerin and lamin C to the nuclear envelope. Here we report the development of lamin C-only mice (Lmna(LCO/LCO)), which produce lamin C but no lamin A or prelamin A (the precursor to lamin A). Lmna(LCO/LCO) mice were entirely healthy, and Lmna(LCO/LCO) cells displayed normal emerin targeting and exhibited only very minimal alterations in nuclear shape and nuclear deformability. Thus, at least in the mouse, prelamin A and lamin A appear to be dispensable. Nevertheless, an accumulation of farnesyl-prelamin A (as occurs with a deficiency in the prelamin A processing enzyme Zmpste24) caused dramatically misshapen nuclei and progeria-like disease phenotypes. The apparent dispensability of prelamin A suggested that lamin A-related progeroid syndromes might be treated with impunity by reducing prelamin A synthesis. Remarkably, the presence of a single Lmna(LCO) allele eliminated the nuclear shape abnormalities and progeria-like disease phenotypes in Zmpste24-/- mice. Moreover, treating Zmpste24-/- cells with a prelamin A-specific antisense oligonucleotide reduced prelamin A levels and significantly reduced the frequency of misshapen nuclei. These studies suggest a new therapeutic strategy for treating progeria and other lamin A diseases.
Figures
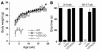




Comment in
-
Good news in the nuclear envelope: loss of lamin A might be a gain.J Clin Invest. 2006 Mar;116(3):632-4. doi: 10.1172/JCI27820. J Clin Invest. 2006. PMID: 16511598 Free PMC article. Review.
References
-
- Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J. Biol. Chem. 1993;268:16321–16326. - PubMed
-
- Muchir A, Worman HJ. The nuclear envelope and human disease. Physiology (Bethesda). 2004;19:309–314. - PubMed
-
- Hutchison CJ, Worman HJ. A-type lamins: guardians of the soma? Nat. Cell Biol. 2004;6:1062–1067. - PubMed
-
- Mounkes LC, Burke B, Stewart CL. The A-type lamins. Nuclear structural proteins as a focus for muscular dystrophy and cardiovascular diseases. Trends Cardiovasc. Med. 2001;11:280–285. - PubMed
-
- Wilson KL. The nuclear envelope, muscular dystrophy and gene expression. Trends Cell Biol. 2000;10:125–129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

