Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature
- PMID: 16511605
- PMCID: PMC1386106
- DOI: 10.1172/JCI25303
Loss of constitutive activity of the growth hormone secretagogue receptor in familial short stature
Abstract
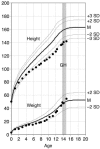
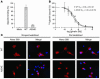
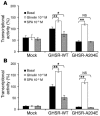
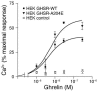
The growth hormone (GH) secretagogue receptor (GHSR) was cloned as the target of a family of synthetic molecules endowed with GH release properties. As shown recently through in vitro means, this receptor displays a constitutive activity whose clinical relevance is unknown. Although pharmacological studies have demonstrated that its endogenous ligand--ghrelin--stimulates, through the GHSR, GH secretion and appetite, the physiological importance of the GHSR-dependent pathways remains an open question that gives rise to much controversy. We report the identification of a GHSR missense mutation that segregates with short stature within 2 unrelated families. This mutation, which results in decreased cell-surface expression of the receptor, selectively impairs the constitutive activity of the GHSR, while preserving its ability to respond to ghrelin. This first description, to our knowledge, of a functionally significant GHSR mutation, which unveils the critical importance of the GHSR-associated constitutive activity, discloses an unusual pathogenic mechanism of growth failure in humans.
Figures


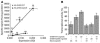
Comment in
-
Ghrelin receptor mutations--too little height and too much hunger.J Clin Invest. 2006 Mar;116(3):637-41. doi: 10.1172/JCI27999. J Clin Invest. 2006. PMID: 16511600 Free PMC article. Review.
References
-
- Howard AD, et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. - PubMed
-
- Smith RG, et al. Peptidomimetic regulation of growth hormone secretion. Endocr. Rev. 1997;18:621–645. - PubMed
-
- Kojima M, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. - PubMed
-
- Cummings DE, et al. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50:1714–1719. - PubMed
-
- Takaya K, et al. Ghrelin strongly stimulates growth hormone release in humans. J. Clin. Endocrinol. Metab. 2000;85:4908–4911. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

