A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma
- PMID: 16511606
- PMCID: PMC1386108
- DOI: 10.1172/JCI26582
A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma
Abstract
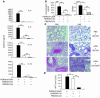
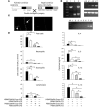
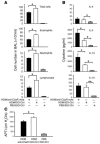
Complement component 5 (C5) has been described as either promoting or protecting against airway hyperresponsiveness (AHR) in experimental allergic asthma, suggesting pleomorphic effects of C5. Here we report that local pharmacological targeting of the C5a receptor (C5aR) prior to initial allergen sensitization in murine models of inhalation tolerance or allergic asthma resulted in either induction or marked enhancement of Th2-polarized immune responses, airway inflammation, and AHR. Importantly, C5aR-deficient mice exhibited a similar, increased allergic phenotype. Pulmonary allergen exposure in C5aR-targeted mice resulted in increased sensitization and accumulation of CD4+ CD69+ T cells associated with a marked increase in pulmonary myeloid, but not plasmacytoid, DC numbers. Pulmonary DCs from C5aR-targeted mice produced large amounts of CC chemokine ligand 17 (CCL17) and CCL22 ex vivo, suggesting a negative impact of C5aR signaling on pulmonary homing of Th2 cells. In contrast, C5aR targeting in sensitized mice led to suppressed airway inflammation and AHR but was still associated with enhanced production of Th2 effector cytokines. These data suggest a dual role for C5a in allergic asthma, i.e., protection from the development of maladaptive type 2 immune responses during allergen sensitization at the DC/T cell interface but enhancement of airway inflammation and AHR in an established inflammatory environment.
Figures




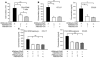
Comment in
-
An unexpected role for the anaphylatoxin C5a receptor in allergic sensitization.J Clin Invest. 2006 Mar;116(3):628-32. doi: 10.1172/JCI27876. J Clin Invest. 2006. PMID: 16511597 Free PMC article. Review.
References
-
- Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu. Rev. Immunol. 1999;17:255–281. - PubMed
-
- Wills-Karp M, Ewart SL. Time to draw breath: asthma-susceptibility genes are identified. Nat. Rev. Genet. 2004;5:376–387. - PubMed
-
- Maruo K, Akaike T, Ono T, Okamoto T, Maeda H. Generation of anaphylatoxins through proteolytic processing of C3 and C5 by house dust mite protease. J. Allergy Clin. Immunol. 1997;100:253–260. - PubMed
-
- Köhl J. Anaphylatoxins and infectious and non-infectious inflammatory diseases. Mol. Immunol. 2001;38:175–187. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

