Ablation of myosin-binding protein-C accelerates force development in mouse myocardium
- PMID: 16513777
- PMCID: PMC1459529
- DOI: 10.1529/biophysj.105.078147
Ablation of myosin-binding protein-C accelerates force development in mouse myocardium
Abstract
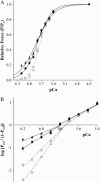
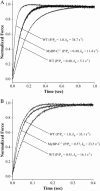
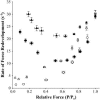
Myosin-binding protein-C (MyBP-C) is a thick filament-associated protein that binds tightly to myosin. Given that cMyBP-C may act to modulate cooperative activation of the thin filament by constraining the availability of myosin cross-bridges for binding to actin, we investigated the role of MyBP-C in the regulation of cardiac muscle contraction. We assessed the Ca(2+) sensitivity of force (pCa(50)) and the activation dependence of the rate of force redevelopment (k(tr)) in skinned myocardium isolated from wild-type (WT) and cMyBP-C null (cMyBP-C(-/-)) mice. Mechanical measurements were performed at 22 degrees C in the absence and presence of a strong-binding, nonforce-generating analog of myosin subfragment-1 (NEM-S1). In the absence of NEM-S1, maximal force and k(tr) and the pCa(50) of isometric force did not differ between WT and cMyBP-C(-/-) myocardium; however, ablation of cMyBP-C-accelerated k(tr) at each submaximal force. Treatment of WT and cMyBP-C(-/-) myocardium with 3 muM NEM-S1 elicited similar increases in pCa(50,) but the effects of NEM-S1 to increase k(tr) at submaximal forces and thereby markedly reduce the activation dependence of k(tr) occurred to a greater degree in cMyBP-C(-/-) myocardium. Together, these results support the idea that cMyBP-C normally acts to constrain the interaction between myosin and actin, which in turn limits steady-state force development and the kinetics of cross-bridge interaction.
Figures

References
-
- Solaro, R. J., and H. M. Rarick. 1998. Troponin and tropomyosin: proteins that switch on and tune in the activity of cardiac myofilaments. Circ. Res. 83:471–480. - PubMed
-
- Lehrer, S. S. 1994. The regulatory switch of the muscle thin filament: Ca2+ or myosin heads. J. Muscle Res. Cell Motil. 15:232–236. - PubMed
-
- Gordon, A. M., E. Homsher, and M. Regnier. 2000. Regulation of contraction in striated muscle. Physiol. Rev. 80:853–924. - PubMed
-
- Johnson, J. D., J. H. Collins, S. P. Robertson, and J. D. Potter. 1980. A fluorescent probe study of Ca2+ binding to the Ca2+ specific sites of cardiac troponin and troponin C. J. Biol. Chem. 255:9635–9640. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

