Force-induced adsorption and anisotropic growth of focal adhesions
- PMID: 16513789
- PMCID: PMC1440730
- DOI: 10.1529/biophysj.105.074377
Force-induced adsorption and anisotropic growth of focal adhesions
Abstract
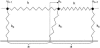
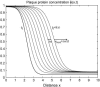
Focal adhesions are micrometer-sized protein aggregates that connect actin stress fibers to the extracellular matrix, a network of macromolecules surrounding tissue cells. The actin fibers are under tension due to actin-myosin contractility. Recent measurements have shown that as the actin force is increased, these adhesions grow in size and in the direction of the force. This is in contrast to the growth of condensed domains of surface-adsorbed molecules in which the dynamics are isotropic. We predict these force-sensitive, anisotropic dynamics of focal adhesions from a model for the adsorption of proteins from the cytoplasm to the adhesion site. Our theory couples the mechanical forces and elasticity to the adsorption dynamics via force-induced conformational changes of molecular-sized mechanosensors located in the focal adhesion. We predict the velocity of both the front and back of the adhesion as a function of the applied force. In addition, our results show that the relative motion of the front and back of the adhesion is asymmetric and in different ranges of forces, the adhesion can either shrink or grow in the direction of the force.
Figures







References
-
- Geiger, B., A. Bershadsky, R. Pankov, and K. M. Yamada. 2001. Transmembrane extracellular matrix-cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2:793–805. - PubMed
-
- Giancotti, F. G., and E. Ruoslahti. 1999. Integrin signaling. Science. 285:1028–1032. - PubMed
-
- Martin, P. 1997. Wound healing: aiming for perfect skin regeneration. Science. 276:75–81. - PubMed
-
- Riveline, D., E. Zamir, N. Q. Balaban, U. S. Schwarz, T. Ishizaki, S. Narumiya, Z. Kam, B. Geiger, and A. D. Bershadsky. 2001. Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mDia1-dependent and ROCK-independent mechanism. J. Cell Biol. 153:1175–1185. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

