G protein betagamma complex translocation from plasma membrane to Golgi complex is influenced by receptor gamma subunit interaction
- PMID: 16517125
- PMCID: PMC2230546
- DOI: 10.1016/j.cellsig.2006.01.016
G protein betagamma complex translocation from plasma membrane to Golgi complex is influenced by receptor gamma subunit interaction
Abstract
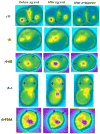
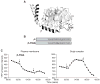
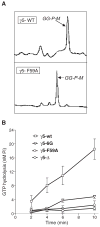
On activation of a receptor the G protein betagamma complex translocates away from the receptor on the plasma membrane to the Golgi complex. The rate of translocation is influenced by the type of gamma subunit associated with the G protein. Complementary approaches--imaging living cells expressing fluorescent protein tagged G proteins and assaying reconstituted receptors and G proteins in vitro--were used to identify mechanisms at the basis of the translocation process. Translocation of Gbetagamma containing mutant gamma subunits with altered prenyl moieties showed that the differences in the prenyl moieties were not sufficient to explain the differential effects of geranylgeranylated gamma5 and farnesylated gamma11 on the translocation process. The translocation properties of Gbetagamma were altered dramatically by mutating the C terminal tail region of the gamma subunit. The translocation characteristics of these mutants suggest that after receptor activation, Gbetagamma retains contact with a receptor through the gamma subunit C terminal domain and that differential interaction of the activated receptor with this domain controls Gbetagamma translocation from the plasma membrane.
Figures



References
-
- Hou Y, Chang V, Capper AB, Taussig R, Gautam N. J Biol Chem. 2001;276(23):19982. - PubMed
-
- McIntire WE, MacCleery G, Garrison JC. J Biol Chem. 2001;276(19):15801. - PubMed
-
- Lim WK, Myung CS, Garrison JC, Neubig RR. Biochemistry. 2001;40(35):10532. - PubMed
-
- Richardson M, Robishaw JD. J Biol Chem. 1999;274(19):13525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

