Rate of over-diagnosis of breast cancer 15 years after end of Malmö mammographic screening trial: follow-up study
- PMID: 16517548
- PMCID: PMC1410836
- DOI: 10.1136/bmj.38764.572569.7C
Rate of over-diagnosis of breast cancer 15 years after end of Malmö mammographic screening trial: follow-up study
Abstract
Objective: To evaluate the rate of over-diagnosis of breast cancer 15 years after the end of the Malmö mammographic screening trial.
Design: Follow-up study.
Setting: Malmö, Sweden.
Subjects: 42 283 women aged 45-69 years at randomisation.
Interventions: Screening for breast cancer with mammography or not (controls). Screening was offered at the end of the randomisation design to both groups aged 45-54 at randomisation but not to groups aged 55-69 at randomisation.
Main outcome measures: Rate of over-diagnosis of breast cancer (in situ and invasive), calculated as incidence in the invited and control groups, during period of randomised design (period 1), during period after randomised design ended (period 2), and at end of follow-up.
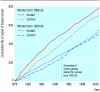
Results: In women aged 55-69 years at randomisation the relative rates of over-diagnosis of breast cancer (95% confidence intervals) were 1.32 (1.14 to 1.53) for period 1, 0.92 (0.79 to 1.06) for period 2, and 1.10 (0.99 to 1.22) at the end of follow-up.
Conclusion: Conclusions on over-diagnosis of breast cancer in the Malmö mammographic screening trial can be drawn mainly for women aged 55-69 years at randomisation whose control groups were never screened. Fifteen years after the trial ended the rate of over-diagnosis of breast cancer was 10% in this age group.
Figures

Comment in
-
Over-diagnosis in breast cancer screening.BMJ. 2006 Mar 25;332(7543):691-2. doi: 10.1136/bmj.38768.401030.7C. Epub 2006 Mar 3. BMJ. 2006. PMID: 16517549 Free PMC article. No abstract available.
-
Evaluating new screening tests for breast cancer.BMJ. 2006 Mar 25;332(7543):678-9. doi: 10.1136/bmj.332.7543.678. BMJ. 2006. PMID: 16565097 Free PMC article. No abstract available.
-
Ramifications of screening for breast cancer: definition of overdiagnosis is confusing in follow-up of Malmö trial.BMJ. 2006 Mar 25;332(7543):727-8. doi: 10.1136/bmj.332.7543.727-b. BMJ. 2006. PMID: 16565134 Free PMC article. No abstract available.
-
Ramifications of screening for breast cancer: overdiagnosis in the Malmö trial was considerably underestimated.BMJ. 2006 Mar 25;332(7543):727. doi: 10.1136/bmj.332.7543.727-a. BMJ. 2006. PMID: 16565135 Free PMC article. No abstract available.
-
Ramifications of screening for breast cancer: 1 in 4 cancers detected by mammography are pseudocancers.BMJ. 2006 Mar 25;332(7543):727. doi: 10.1136/bmj.332.7543.727. BMJ. 2006. PMID: 16565136 Free PMC article. No abstract available.
-
Ramifications of screening for breast cancer: more debate and better information still needed.BMJ. 2006 Mar 25;332(7543):728. doi: 10.1136/bmj.332.7543.728-a. BMJ. 2006. PMID: 16565138 Free PMC article. No abstract available.
-
Ramifications of screening for breast cancer: consent for screening.BMJ. 2006 Mar 25;332(7543):728. doi: 10.1136/bmj.332.7543.728. BMJ. 2006. PMID: 16565139 Free PMC article. No abstract available.
References
-
- Baines CJ. Are there downsides to mammography screening? Breast J 2005;11: S7-10. - PubMed
-
- Duffy SW. Some current issues in breast cancer screening. J Med Screen 2005;12: 128-33. - PubMed
-
- Paci E, Warwick J, Falini P, Duffy SW. Overdiagnosis in screening: is the increase in breast cancer incidence rates a cause for concern? J Med Screen 2004;11: 23-7. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
