Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm
- PMID: 16517655
- PMCID: PMC1393226
- DOI: 10.1128/AEM.72.3.2064-2069.2006
Chelator-induced dispersal and killing of Pseudomonas aeruginosa cells in a biofilm
Abstract
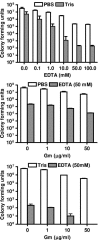
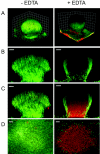
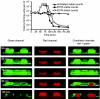
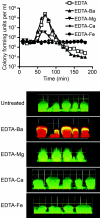
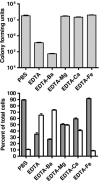
Biofilms consist of groups of bacteria attached to surfaces and encased in a hydrated polymeric matrix. Bacteria in biofilms are more resistant to the immune system and to antibiotics than their free-living planktonic counterparts. Thus, biofilm-related infections are persistent and often show recurrent symptoms. The metal chelator EDTA is known to have activity against biofilms of gram-positive bacteria such as Staphylococcus aureus. EDTA can also kill planktonic cells of Proteobacteria like Pseudomonas aeruginosa. In this study we demonstrate that EDTA is a potent P. aeruginosa biofilm disrupter. In Tris buffer, EDTA treatment of P. aeruginosa biofilms results in 1,000-fold greater killing than treatment with the P. aeruginosa antibiotic gentamicin. Furthermore, a combination of EDTA and gentamicin results in complete killing of biofilm cells. P. aeruginosa biofilms can form structured mushroom-like entities when grown under flow on a glass surface. Time lapse confocal scanning laser microscopy shows that EDTA causes a dispersal of P. aeruginosa cells from biofilms and killing of biofilm cells within the mushroom-like structures. An examination of the influence of several divalent cations on the antibiofilm activity of EDTA indicates that magnesium, calcium, and iron protect P. aeruginosa biofilms against EDTA treatment. Our results are consistent with a mechanism whereby EDTA causes detachment and killing of biofilm cells.
Figures
References
-
- Ayres, H. M., J. R. Furr, and A. D. Russell. 1998. Effect of divalent cations on permeabilizer-induced lysozyme lysis of Pseudomonas aeruginosa. Lett. Appl. Microbiol. 27:372-374. - PubMed
-
- Ayres, H. M., J. R. Furr, and A. D. Russell. 1999. Effect of permeabilizers on antibiotic sensitivity of Pseudomonas aeruginosa. Lett. Appl. Microbiol. 28:13-16. - PubMed
-
- Ayres, H. M., D. N. Payne, J. R. Furr, and A. D. Russell. 1998. Effect of permeabilizing agents on antibacterial activity against a simple Pseudomonas aeruginosa biofilm. Lett. Appl. Microbiol. 27:79-82. - PubMed
-
- Boggis, W., M. A. Kenward, and M. R. Brown. 1979. Effects of divalent metal cations in the growth medium upon sensitivity of batch-grown Pseudomonas aeruginosa to EDTA or polymyxin B. J. Appl. Bacteriol. 47:477-488. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

