Downregulation of the Drosophila immune response by peptidoglycan-recognition proteins SC1 and SC2
- PMID: 16518472
- PMCID: PMC1383489
- DOI: 10.1371/journal.ppat.0020014
Downregulation of the Drosophila immune response by peptidoglycan-recognition proteins SC1 and SC2
Abstract
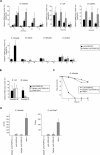
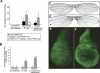
Peptidoglycan-recognition proteins (PGRPs) are evolutionarily conserved molecules that are structurally related to bacterial amidases. Several Drosophila PGRPs have lost this enzymatic activity and serve as microbe sensors through peptidoglycan recognition. Other PGRP family members, such as Drosophila PGRP-SC1 or mammalian PGRP-L, have conserved the amidase function and are able to cleave peptidoglycan in vitro. However, the contribution of these amidase PGRPs to host defense in vivo has remained elusive so far. Using an RNA-interference approach, we addressed the function of two PGRPs with amidase activity in the Drosophila immune response. We observed that PGRP-SC1/2-depleted flies present a specific over-activation of the IMD (immune deficiency) signaling pathway after bacterial challenge. Our data suggest that these proteins act in the larval gut to prevent activation of this pathway following bacterial ingestion. We further show that a strict control of IMD-pathway activation is essential to prevent bacteria-induced developmental defects and larval death.
Conflict of interest statement
Figures
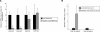


References
-
- Bulet P, Charlet M, Hetru C. Antimicrobial peptides in insect immunity. In: Ezekowitz RAB, Hoffmann JA, editors. Infectious disease: Innate immunity. Totowa (New Jersey): Human Press; 2002. pp. 89–107.
-
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. - PubMed
-
- Hoffmann JA. The immune response of Drosophila . Nature. 2003;426:33–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

