Comparative aspects of cerebral cortical development
- PMID: 16519657
- PMCID: PMC1931431
- DOI: 10.1111/j.1460-9568.2006.04611.x
Comparative aspects of cerebral cortical development
Abstract
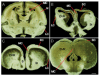
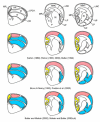
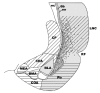
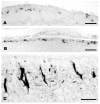
This review aims to provide examples of how both comparative and genetic analyses contribute to our understanding of the rules for cortical development and evolution. Genetic studies have helped us to realize the evolutionary rules of telencephalic organization in vertebrates. The control of the establishment of conserved telencephalic subdivisions and the formation of boundaries between these subdivisions has been examined and the very specific alterations at the striatocortical junction have been revealed. Comparative studies and genetic analyses both demonstrate the differential origin and migratory pattern of the two basic neuron types of the cerebral cortex. GABAergic interneurons are mostly generated in the subpallium and a common mechanism governs their migration to the dorsal cortex in both mammals and sauropsids. The pyramidal neurons are generated within the cortical germinal zone and migrate radially, the earliest generated cell layers comprising preplate cells. Reelin-positive Cajal-Retzius cells are a general feature of all vertebrates studied so far; however, there is a considerable amplification of the Reelin signalling with cortical complexity, which might have contributed to the establishment of the basic mammalian pattern of cortical development. Based on numerous recent observations we shall present the argument that specialization of the mitotic compartments may constitute a major drive behind the evolution of the mammalian cortex. Comparative developmental studies have revealed distinct features in the early compartments of the developing macaque brain, drawing our attention to the limitations of some of the current model systems for understanding human developmental abnormalities of the cortex. Comparative and genetic aspects of cortical development both reveal the workings of evolution.
Figures



References
-
- Anderson SA, Qiu M, Bulfone A, Eisenstat DD, Meneses J, Pedersen R, Rubenstein JL. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997;19(1):27–37. - PubMed
-
- Anderson SA, Kaznowski CE, Horn C, Rubenstein JL, McConnell SK. Distinct origins of neocortical projection neurons and interneurons in vivo. Cereb Cortex. 2002;12:702–709. - PubMed
-
- Angevine JB, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192: 766–768. - PubMed
-
- Arimatsu Y. Latexin: a molecular marker for regional specification in the neocortex. Neurosci Res. 1994;20(2):131–5. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

