Endogenous opioids upregulate brain-derived neurotrophic factor mRNA through delta- and micro-opioid receptors independent of antidepressant-like effects
- PMID: 16519663
- PMCID: PMC1462954
- DOI: 10.1111/j.1460-9568.2006.04621.x
Endogenous opioids upregulate brain-derived neurotrophic factor mRNA through delta- and micro-opioid receptors independent of antidepressant-like effects
Abstract
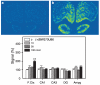
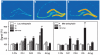
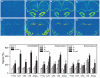
Systemic administration of delta-opioid receptor (DOR) agonists decreases immobility in the forced swim test (FST) and increases brain-derived neurotrophic factor (BDNF) mRNA expression in rats, indicating that DOR agonists may have antidepressant-like effects. The aim of this study was to investigate the effects of central administration of endogenous opioid peptides on behavior in the FST and on brain BDNF mRNA expression in rats. Effects of endogenous opioids were compared with those produced by intracerebroventricular administration of a selective non-peptidic DOR agonist (+)BW373U86. Antidepressant-like effects were measured by decreased immobility in the FST. BDNF mRNA expression was determined by in situ hybridization. Centrally administered (+)BW373U86 decreased immobility and increased BDNF mRNA expression in the frontal cortex through a DOR-mediated mechanism, because these effects were blocked by the DOR antagonist naltrindole, but not by the micro-opioid receptor (MOR) antagonist naltrexone (NTX) or the kappa-opioid receptor antagonist nor-binaltorphimine. Of all the endogenous opioids tested, only leu- and met-enkephalin produced behavioral effects like those of (+)BW373U86 in the FST. Unlike (+)BW373U86, the enkephalins upregulated BDNF mRNA expression in the hippocampus through DOR- and MOR-mediated mechanisms. beta-Endorphin, endomorphin-1 and endomorphin-2 significantly increased BDNF mRNA levels in the frontal cortex, hippocampus and amygdala without reducing immobility; and most of these effects were reversed by NTX. This study is the first to provide evidence that endogenous opioids can upregulate BDNF mRNA expression through the DOR and MOR, and that leu- and met-enkephalin have similar pharmacological profiles to synthetic DOR agonists in producing antidepressant-like effects.
Figures





References
-
- Baamonde A, Dauge V, Ruiz-Gayo M, Fulga IG, Turcaud S, Fournie-Zaluski MC, Ronques BP. Antidepressant-type effects of endogenous enkephalins protected by systemic RB101 are mediated by opioid delta and dopamine D1 receptor stimulation. Eur. J. Pharmacol. 1992;216:157–166. - PubMed
-
- Bengzon J, Kokaia Z, Ernfors P, Kokaia M, Leanza G, Nilsson OG, Persson H, Lindvall O. Regulation of neurotrophin and trkA, trkB and trkC tyrosine kinase receptor messenger RNA expression in kindling. Neuroscience. 1993;53:433–446. - PubMed
-
- Bhattacharya SK, Saraswati M, Sen AP. Effect of centrally administered enkephalins on carrageenan-induced paw oedema in rats. Res. Exp. Med. 1992;192:443–449. - PubMed
-
- Bishop MJ, McNutt RW. An efficient synthesis of the benahydrylpiperazine delta opioid agonist (+)-BW373U86. Bioorg. Med. Chem. Lett. 1995;5:1311–1314.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

