Epithelial cells are sensitive detectors of bacterial pore-forming toxins
- PMID: 16520379
- PMCID: PMC1586115
- DOI: 10.1074/jbc.M511431200
Epithelial cells are sensitive detectors of bacterial pore-forming toxins
Abstract
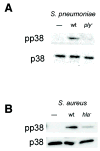
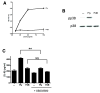
Epithelial cells act as an interface between human mucosal surfaces and the surrounding environment. As a result, they are responsible for the initiation of local immune responses, which may be crucial for prevention of invasive infection. Here we show that epithelial cells detect the presence of bacterial pore-forming toxins (including pneumolysin from Streptococcus pneumoniae, alpha-hemolysin from Staphylococcus aureus, streptolysin O from Streptococcus pyogenes, and anthrolysin O from Bacillus anthracis) at nanomolar concentrations, far below those required to cause cytolysis. Phosphorylation of p38 MAPK appears to be a conserved response of epithelial cells to subcytolytic concentrations of bacterial poreforming toxins, and this activity is inhibited by the addition of high molecular weight osmolytes to the extracellular medium. By sensing osmotic stress caused by the insertion of a sublethal number of pores into their membranes, epithelial cells may act as an early warning system to commence an immune response, while the local density of toxin-producing bacteria remains low. Osmosensing may thus represent a novel innate immune response to a common bacterial virulence strategy.
Figures


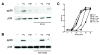


References
-
- Burns DL. ASM Press; Washington, D.C: 2003. Bacterial protein toxins.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

