UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation
- PMID: 16520734
- PMCID: PMC2613020
- DOI: 10.1038/nn1666
UNC-6/Netrin induces neuronal asymmetry and defines the site of axon formation
Abstract
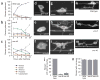
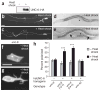
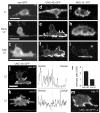
UNC-6/Netrin and its receptor UNC-40/DCC are conserved regulators of growth cone guidance. By directly observing developing neurons in vivo, we show that UNC-6 and UNC-40 also function during axon formation to initiate, maintain and orient asymmetric neuronal growth. The immature HSN neuron of Caenorhabditis elegans breaks spherical symmetry to extend a leading edge toward ventral UNC-6. In unc-6 and unc-40 mutants, leading edge formation fails, the cell remains symmetrical until late in development and the axon that eventually forms is misguided. Thus netrin has two activities: one that breaks neuronal symmetry and one that guides the future axon. As the axon forms, UNC-6, UNC-40 and the lipid modulators AGE-1/phosphoinositide 3-kinase (PI3K) and DAF-18/PTEN drive the actin-regulatory pleckstrin homology (PH) domain protein MIG-10/lamellipodin ventrally in HSN to promote asymmetric growth. The coupling of a directional netrin cue to sustained asymmetric growth via PI3K signaling is reminiscent of polarization in chemotaxing cells.
Figures



References
-
- Inagaki N, et al. CRMP-2 induces axons in cultured hippocampal neurons. Nat Neurosci. 2001;4:781–782. - PubMed
-
- Shi SH, Cheng T, Jan LY, Jan YN. APC and GSK-3β are involved in mPar3 targeting to the nascent axon and establishment of neuronal polarity. Curr Biol. 2004;14:2025–2032. - PubMed
-
- Kishi M, Pan YA, Crump JG, Sanes JR. Mammalian SAD kinases are required for neuronal polarization. Science. 2005;307:929–932. - PubMed
-
- Shi SH, Jan LY, Jan YN. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell. 2003;112:63–75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

