A role for ion channels in glioma cell invasion
- PMID: 16520829
- PMCID: PMC1389710
- DOI: 10.1017/S17440925X06000044
A role for ion channels in glioma cell invasion
Abstract
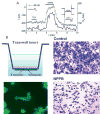
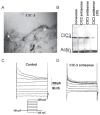
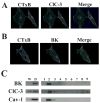
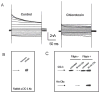
Many cells, including neuronal and glial progenitor cells, stem cells and microglial cells, have the capacity to move through the extracellular spaces of the developing and mature brain. This is particularly pronounced in astrocyte-derived tumors, gliomas, which diffusely infiltrate the normal brain. Although a significant body of literature exists regarding signals that are involved in the guidance of cells and their processes, little attention has been paid to cell-shape and cell-volume changes of migratory cells. However, extracellular spaces in the brain are very narrow and represent a major obstacle that requires cells to dynamically regulate their volume. Recent studies in glioma cells show that this involves the secretion of Cl(-) and K(+) with water. Pharmacological inhibition of Cl(-) channels impairs their ability to migrate and limits tumor progression in experimental tumor models. One Cl(-)-channel inhibitor, chlorotoxin, is currently in Phase II clinical trials to treat malignant glioma. This article reviews our current knowledge of cell-volume changes and the role of ion channels during the migration of glioma cells. It also discusses evidence that supports the importance of channel-mediated cell-volume changes in the migration of immature neurons and progenitor cells during development. New unpublished data is presented, which demonstrates that Cl(-) and K(+) channels involved in cell shrinkage localize to lipid-raft domains on the invadipodia of glioma cells and that their presence might be regulated by trafficking of these proteins in and out of lipid rafts.
Figures


Similar articles
-
An unexpected role for ion channels in brain tumor metastasis.Exp Biol Med (Maywood). 2008 Jul;233(7):779-91. doi: 10.3181/0711-MR-308. Epub 2008 Apr 29. Exp Biol Med (Maywood). 2008. PMID: 18445774 Free PMC article. Review.
-
Modulation of glioma cell migration and invasion using Cl(-) and K(+) ion channel blockers.J Neurosci. 1999 Jul 15;19(14):5942-54. doi: 10.1523/JNEUROSCI.19-14-05942.1999. J Neurosci. 1999. PMID: 10407033 Free PMC article.
-
Cl- and K+ channels and their role in primary brain tumour biology.Philos Trans R Soc Lond B Biol Sci. 2014 Feb 3;369(1638):20130095. doi: 10.1098/rstb.2013.0095. Print 2014 Mar 19. Philos Trans R Soc Lond B Biol Sci. 2014. PMID: 24493743 Free PMC article. Review.
-
Ion channels and transporters [corrected] in cancer. 2. Ion channels and the control of cancer cell migration.Am J Physiol Cell Physiol. 2011 Sep;301(3):C541-9. doi: 10.1152/ajpcell.00102.2011. Epub 2011 May 4. Am J Physiol Cell Physiol. 2011. PMID: 21543740 Free PMC article. Review.
-
Calcium entry via TRPC1 channels activates chloride currents in human glioma cells.Cell Calcium. 2013 Mar;53(3):187-94. doi: 10.1016/j.ceca.2012.11.013. Epub 2012 Dec 20. Cell Calcium. 2013. PMID: 23261316 Free PMC article.
Cited by
-
TRP Channels in Brain Tumors.Front Cell Dev Biol. 2021 Apr 13;9:617801. doi: 10.3389/fcell.2021.617801. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33928077 Free PMC article. Review.
-
Structure-Activity Relationship of Chlorotoxin-Like Peptides.Toxins (Basel). 2016 Feb 2;8(2):36. doi: 10.3390/toxins8020036. Toxins (Basel). 2016. PMID: 26848686 Free PMC article.
-
Nanoparticles for Targeting Intratumoral Hypoxia: Exploiting a Potential Weakness of Glioblastoma.Pharm Res. 2016 Sep;33(9):2059-77. doi: 10.1007/s11095-016-1947-8. Epub 2016 May 26. Pharm Res. 2016. PMID: 27230936 Review.
-
A C-Terminal Fragment of Chlorotoxin Retains Bioactivity and Inhibits Cell Migration.Front Pharmacol. 2019 Mar 20;10:250. doi: 10.3389/fphar.2019.00250. eCollection 2019. Front Pharmacol. 2019. PMID: 30949052 Free PMC article.
-
Cytotoxic Effects of two Iranian Scorpions Odontobuthusdoriae and Bothutus saulcyi on Five Human Cultured Cell lines and Fractions of Toxic Venom.Iran J Pharm Res. 2012 Winter;11(1):357-67. Iran J Pharm Res. 2012. PMID: 24250459 Free PMC article.
References
-
- Ben Ari Y, Khalilov I, Represa A, Gozlan H. Interneurons set the tune of developing networks. Trends in Neurosciences. 2004;27:422–427. - PubMed
-
- Berger T, Walz W, Schnitzer J, Kettenmann H. GABA- and glutamateactivated currents in glial cells of the mouse corpus callosum slice. Journal of Neuroscience Research. 1992;31:21–27. - PubMed
-
- Bordey A, Sontheimer H, Trouslard J. Muscarinic activation of bk channels induces membrane oscillations in glioma cells and leads to inhibition of cell migration. Journal of Membrane Biology. 2000;176:31–40. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
