Epidural anaesthesia restores pancreatic microcirculation and decreases the severity of acute pancreatitis
- PMID: 16521220
- PMCID: PMC4066157
- DOI: 10.3748/wjg.v12.i6.915
Epidural anaesthesia restores pancreatic microcirculation and decreases the severity of acute pancreatitis
Abstract
Aim: To investigate the effect of epidural anaesthesia (EA) on pancreatic microcirculation during acute pancreatitis (AP).
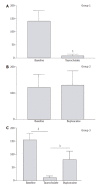
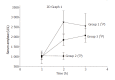
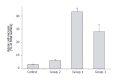
Methods: AP was induced by injection of sodium taurocholate into the pancreatic duct of Sprague-Dawley rats. To realize EA, a catheter was introduced into the epidural space between T7 and T9 and bupivacaine was injected. Microcirculatory flow was measured by laser Doppler flowmetry. Arterial blood gas analyses were performed. At the end of the experiment (<or=5 h), pancreas was removed for histology. The animals were divided into three groups: Group 1 (n=9), AP without EA; Group 2 (n=4), EA without AP; and Group 3 (n=6), AP treated by EA.
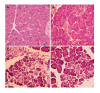
Results: In Group 1, pancreatic microcirculatory flow prior to AP was 141+/- 39 perfusion units (PU). After AP, microcirculatory flow obviously decreased to 9+/- 6 PU (P<0.05). Metabolic acidosis developed with base excess (BE) of -14+/-3 mmol/L. Histology revealed extensive edema and tissue necrosis. In Group 2, EA did not significantly modify microcirculatory flow. BE remained unchanged and histological analysis showed normal pancreatic tissue. In Group 3, AP initially caused a significant decrease in microcirculatory flow from 155+/-25 to 11+/-7 PU (P<0.05). After initiation of EA, microcirculatory flow obviously increased again to 81+/-31 PU (P<0.05). BE was -6+/-4 mmol/L, which was significantly different compared to Group 1 (P<0.05). Furthermore, histology revealed less extensive edema and necrosis in pancreatic tissue in Group 3 than that in Group 1.
Conclusion: AP caused dramatic microcirculatory changes within the pancreas, with development of metabolic acidosis and tissue necrosis. EA allowed partial restoration of microcirculatory flow and prevented development of tissue necrosis and systemic complications. Therefore, EA should be considered as therapeutic option to prevent evolution from edematous to necrotic AP.
Figures



References
-
- Beger HG, Rau B, Isenmann R. Prevention of severe change in acute pancreatitis: prediction and prevention. J Hepatobiliary Pancreat Surg. 2001;8:140–147. - PubMed
-
- Bradley EL 3rd. A clinically based classification system for acute pancreatitis. Summary of the International Symposium on Acute Pancreatitis, Atlanta, Ga, September 11 through 13, 1992. Arch Surg. 1993;128:586–590. - PubMed
-
- Plusczyk T, Westermann S, Rathgeb D, Feifel G. Acute pancreatitis in rats: effects of sodium taurocholate, CCK-8, and Sec on pancreatic microcirculation. Am J Physiol. 1997;272:G310–G320. - PubMed
-
- Dobosz M, Hac S, Mionskowska L, Dobrowolski S, Wajda Z. Microcirculatory disturbances of the pancreas in cerulein-induced acute pancreatitis in rats with reference to L-arginine, heparin, and procaine treatment. Pharmacol Res. 1997;36:123–128. - PubMed
-
- Klar E, Schratt W, Foitzik T, Buhr H, Herfarth C, Messmer K. Impact of microcirculatory flow pattern changes on the development of acute edematous and necrotizing pancreatitis in rabbit pancreas. Dig Dis Sci. 1994;39:2639–2644. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

