The aquaporins
- PMID: 16522221
- PMCID: PMC1431727
- DOI: 10.1186/gb-2006-7-2-206
The aquaporins
Abstract
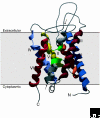
Water is the major component of all living cells, and efficient regulation of water homeostasis is essential for many biological processes. The mechanism by which water passes through biological membranes was a matter of debate until the discovery of the aquaporin water channels. Aquaporins are intrinsic membrane proteins characterized by six transmembrane helices that selectively allow water or other small uncharged molecules to pass along the osmotic gradient. In addition, recent observations show that some aquaporins also facilitate the transport of volatile substances, such as carbon dioxide (CO2) and ammonia (NH3), across membranes. Aquaporins usually form tetramers, with each monomer defining a single pore. Aquaporin-related proteins are found in all organisms, from archaea to mammals. In both uni- and multicellular organisms, numerous isoforms have been identified that are differentially expressed and modified by post-translational processes, thus allowing fine-tuned tissue-specific osmoregulation. In mammals, aquaporins are involved in multiple physiological processes, including kidney and salivary gland function. They are associated with several clinical disorders, such as kidney dysfunction, loss of vision and brain edema.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

