Control of small inhibitory RNA levels and RNA interference by doxycycline induced activation of a minimal RNA polymerase III promoter
- PMID: 16522642
- PMCID: PMC1390691
- DOI: 10.1093/nar/gkl034
Control of small inhibitory RNA levels and RNA interference by doxycycline induced activation of a minimal RNA polymerase III promoter
Abstract
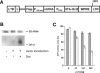
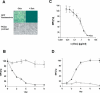
RNA interference (RNAi) mediated by expression of short hairpin RNAs (shRNAs) is a powerful tool for efficiently suppressing target genes. The approach allows studies of the function of individual genes and may also be applied to human therapy. However, in many instances regulation of RNAi by administration of a small inducer molecule will be required. To date, the development of appropriate regulatory systems has been hampered by the few possibilities for modification within RNA polymerase III promoters capable of driving efficient expression of shRNAs. We have developed an inducible minimal RNA polymerase III promoter that is activated by a novel recombinant transactivator in the presence of doxycycline (Dox). The recombinant transactivator and the engineered promoter together form a system permitting regulation of RNAi by Dox-induced expression of shRNAs. Regulated RNAi was mediated by one single lentiviral vector, blocked the expression of green fluorescent protein (GFP) in a GFP-expressing HEK 293T derived cell line and suppressed endogenous p53 in wild-type HEK 293T, MCF-7 and A549 cells. RNA interference was induced in a dose- and time-dependent manner by administration of Dox, silenced the expression of both target genes by 90% and was in particular reversible after withdrawal of Dox.
Figures


References
-
- Hannon G.J. RNA interference. Nature. 2002;418:244–251. - PubMed
-
- Brummelkamp T.R., Bernards R., Agami R. A system for stable expression of short interfering RNAs in mammalian cells. Science. 2002;296:550–553. - PubMed
-
- Xia H., Mao Q., Paulson H.L., Davidson B.L. siRNA mediated gene silencing in vitro and in vivo. Nat. Biotechnol. 2002;20:1006–1010. - PubMed
-
- Abbas-Terki T., Blanco-Bose W., Déglon N., Pralong W., Aebischer P. Lentiviral-mediated RNA interference. Hum. Gene Ther. 2002;13:2197–2201. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

