Endotoxin contamination in commercially available pokeweed mitogen contributes to the activation of murine macrophages and human dendritic cell maturation
- PMID: 16522770
- PMCID: PMC1391957
- DOI: 10.1128/CVI.13.3.309-313.2006
Endotoxin contamination in commercially available pokeweed mitogen contributes to the activation of murine macrophages and human dendritic cell maturation
Abstract
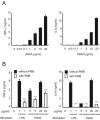
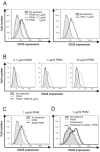
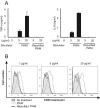
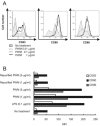
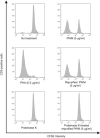
Commercially available pokeweed mitogen (PWM) has been reported to activate macrophages, leading to production of proinflammatory cytokines and nitric oxide (NO). However, we found that polymyxin B (PMB), a specific inhibitor of endotoxin activity, inhibited the PWM-induced expression of proinflammatory cytokines and NO and the activation of Toll-like receptor 4 (TLR4). A kinetic-turbidimetric Limulus amebocyte lysate assay demonstrated that commercial PWM contained substantial endotoxin, over 10(4) endotoxin units/mg of the PWM. A PWM repurified by PMB-coupled beads no longer induced the expression of proinflammatory cytokines, TLR4 activation, or dendritic cell maturation. However, the repurified PWM remained able to induce proliferation of human lymphocytes, which is a representative characteristic of PWM. These results suggest that commercial PWM might be contaminated with a large amount of endotoxin, resulting in the attribution of misleading immunological properties to PWM.
Figures
References
-
- Bang, F. B. 1956. A bacterial disease of Limulus polyphemus. Bull. Johns Hopkins Hosp. 98:325-351. - PubMed
-
- Bessler, W. G., and U. Henning. 1979. Protein I and protein II from the outer membrane of Escherichia coli are mouse B-lymphocyte mitogens. Z. Immunitaetsforsch. Immunobiol. 155:387-398. - PubMed
-
- Chow, J. C., D. W. Young, D. T. Golenbock, W. J. Christ, and F. Gusovsky. 1999. Toll-like receptor-4 mediates lipopolysaccharide-induced signal transduction. J. Biol. Chem. 274:10689-10692. - PubMed
-
- Cohen, M. S., J. Mao, G. T. Rasmussen, J. S. Serody, and B. E. Britigan. 1992. Interaction of lactoferrin and lipopolysaccharide (LPS): effects on the antioxidant property of lactoferrin and the ability of LPS to prime human neutrophils for enhanced superoxide formation. J. Infect. Dis. 166:1375-1378. - PubMed
-
- Coyne, C. P., J. T. Moritz, and V. C. Langston. 1994. Semi-synthesis of polymyxin-B conjugated ovalbumin: evaluation of lipopolysaccharide binding avidity and neutralization of induced tnf-alpha synthesis. Biotherapy 8:69-83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

