NMR study of the tetrameric KcsA potassium channel in detergent micelles
- PMID: 16522799
- PMCID: PMC2242490
- DOI: 10.1110/ps.051954706
NMR study of the tetrameric KcsA potassium channel in detergent micelles
Abstract
Nuclear magnetic resonance (NMR) studies of large membrane-associated proteins are limited by the difficulties in preparation of stable protein-detergent mixed micelles and by line broadening, which is typical of these macroassemblies. We have used the 68-kDa homotetrameric KcsA, a thermostable N-terminal deletion mutant of a bacterial potassium channel from Streptomyces lividans, as a model system for applying NMR methods to membrane proteins. Optimization of measurement conditions enabled us to perform the backbone assignment of KcsA in SDS micelles and establish its secondary structure, which was found to closely agree with the KcsA crystal structure. The C-terminal cytoplasmic domain, absent in the original structure, contains a 14-residue helix that could participate in tetramerization by forming an intersubunit four-helix bundle. A quantitative estimate of cross- relaxation between detergent and KcsA backbone amide protons, together with relaxation and light scattering data, suggests SDS-KcsA mixed micelles form an oblate spheroid with approximately 180 SDS molecules per channel. K(+) ions bind to the micelle-solubilized channel with a K(D) of 3 +/- 0.5 mM, resulting in chemical shift changes in the selectivity filter. Related pH-induced changes in chemical shift along the "outer" transmembrane helix and the cytoplasmic membrane interface hint at a possible structural explanation for the observed pH-gating of the potassium channel.
Figures





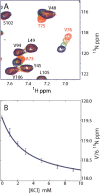


References
-
- Arora A. and Tamm L.K. 2001. Biophysical approaches to membrane protein structure determination Curr. Opin. Struct. Biol. 11: 540–547. - PubMed
-
- Arora A., Abildgaard F., Bushweller J.H., Tamm L.K. 2001. Structure of outer membrane protein A transmembrane domain by NMR spectroscopy Nat. Struct. Biol. 8: 334–338. - PubMed
-
- Bax A. and Grzesiek S. 1993. Methodological advances in protein NMR Acc. Chem. Res. 26: 131–138.
-
- Cai M., Huang Y., Sakaguchi K., Clore G.M., Gronenborn A.M., Craigie R. 1998. An efficient and cost-effective isotope labeling protocol for proteins expressed in Escherichia coli J. Biomol. NMR 11: 97–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

