Fentanyl plasma levels after modified ultrafiltration in infant heart surgery
- PMID: 16524154
- PMCID: PMC4680828
Fentanyl plasma levels after modified ultrafiltration in infant heart surgery
Abstract
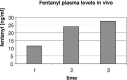
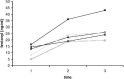
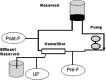
Modified ultrafiltration (MUF) is a novel application of ultrafiltration to remove excess water after cardiopulmonary bypass in pediatric patients that was first reported in 1991. It has gained widespread use as an important adjunct to fluid management in neonates, infants, and pediatric cardiac surgery patients. Now more than two decades after its original description, the exact mechanism of action and effects of this therapy are still a matter of discussion. The aim of this study was to determine the effects of MUF on plasma fentanyl levels using a two-phase in vitro and in vivo study. We designed an in vitro experimental model to simulate MUF that allowed measurement of plasma fentanyl levels while eliminating biologic variables. Plasma fentanyl levels were measured during five consecutive operations on neonates and infants undergoing repair of congenital heart defects. Increases in plasma fentanyl levels were found in vitro as well as in vivo. Fentanyl plasma levels more than doubled after MUF (increased by a factor of 2.22, from 12.4 to 27.5 ng/ml in vivo). The increase in plasma fentanyl levels needs to be taken into account when delivering anesthetic care and when analyzing the effect of MUF on outcome variables.
Conflict of interest statement
The senior author has stated that authors have reported no material, financial or other relationship with any healthcare-related business or other entity whose products or services are discussed in this paper.
Figures
Similar articles
-
Effect of modified ultrafiltration in high-risk patients undergoing operations for congenital heart disease.Ann Thorac Surg. 1998 Sep;66(3):821-7; discussion 828. doi: 10.1016/s0003-4975(98)00606-7. Ann Thorac Surg. 1998. PMID: 9768937 Clinical Trial.
-
The effect of modified ultrafiltration on the postoperative course in patients with congenital heart disease.Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2003;6:128-39. doi: 10.1053/pcsu.2003.50006. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2003. PMID: 12740779 Review.
-
The benefits of continuous ultrafiltration in pediatric cardiac surgery.Scand Cardiovasc J. 2004 Oct;38(5):307-11. doi: 10.1080/14017430410021480. Scand Cardiovasc J. 2004. PMID: 15513315 Clinical Trial.
-
Effect of modified ultrafiltration on plasma thromboxane B2, leukotriene B4, and endothelin-1 in infants undergoing cardiopulmonary bypass.Ann Thorac Surg. 1999 Oct;68(4):1369-75. doi: 10.1016/s0003-4975(99)00978-9. Ann Thorac Surg. 1999. PMID: 10543508
-
Current ultrafiltration techniques before, during and after pediatric cardiopulmonary bypass procedures.Perfusion. 2012 Sep;27(5):438-46. doi: 10.1177/0267659112450061. Epub 2012 Jun 1. Perfusion. 2012. PMID: 22661382 Review.
Cited by
-
Opioids and clonidine modulate cytokine production and opioid receptor expression in neonatal immune cells.J Perinatol. 2013 May;33(5):374-82. doi: 10.1038/jp.2012.124. Epub 2012 Oct 4. J Perinatol. 2013. PMID: 23047422 Free PMC article.
-
In vitro drug adsorption and plasma free hemoglobin levels associated with hollow fiber oxygenators in the extracorporeal life support (ECLS) circuit.J Extra Corpor Technol. 2007 Dec;39(4):234-7. J Extra Corpor Technol. 2007. PMID: 18293808 Free PMC article.
-
Correction to: Pharmacokinetics of Fentanyl and Its Derivatives in Children: A Comprehensive Review.Clin Pharmacokinet. 2018 Mar;57(3):393-417. doi: 10.1007/s40262-017-0609-2. Clin Pharmacokinet. 2018. PMID: 29178007
-
Modified surface coatings and their effect on drug adsorption within the extracorporeal life support circuit.J Extra Corpor Technol. 2010 Sep;42(3):199-202. J Extra Corpor Technol. 2010. PMID: 21114222 Free PMC article.
-
Pharmacokinetics of Fentanyl and Its Derivatives in Children: A Comprehensive Review.Clin Pharmacokinet. 2018 Feb;57(2):125-149. doi: 10.1007/s40262-017-0569-6. Clin Pharmacokinet. 2018. PMID: 28688027 Free PMC article. Review.
References
-
- Naik SK, Knight A, Elliott MJ.. A successful modification of ultrafiltration for cardiopulmonary bypass in children. Perfusion. 1991;6:41–50. - PubMed
-
- Bando K, Turrentine MW, Palaniswamy V, et al. . Effect of modified ultrafiltration in high-risk patients undergoing operations for congenital heart surgery. Ann Thorac Surg. 1998;66:821–8. - PubMed
-
- Draaisma AM, Hazekamp MG, Frank M, et al. . Modified ultrafiltration after cardiopulmonary bypass in pediatric cardiac surgery. Ann Thorac Surg. 1997;64:521–5. - PubMed
-
- Naik SK, Knight A, Elliott M.. A prospective randomized study of a modified technique of ultrafiltration during pediatric open-heart surgery. Circulation. 1991;84(Suppl III):422–31. - PubMed
-
- Wang M-J, Chiu I-S, Hsu C-M, et al. . Efficacy of ultrafiltration in removing inflammatory mediators during pediatric cardiac operations. Ann Thorac Surg. 1996;61:651–6. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
