Optical imaging of luminescence for in vivo quantification of gene electrotransfer in mouse muscle and knee
- PMID: 16524461
- PMCID: PMC1431530
- DOI: 10.1186/1472-6750-6-16
Optical imaging of luminescence for in vivo quantification of gene electrotransfer in mouse muscle and knee
Abstract
Background: Optical imaging is an attractive non-invasive way to evaluate the expression of a transferred DNA, mainly thanks to its lower cost and ease of realization. In this study optical imaging was evaluated for monitoring and quantification of the mouse knee joint and tibial cranial muscle electrotransfer of a luciferase encoding plasmid. Optical imaging was applied to study the kinetics of luciferase expression in both tissues.
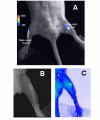
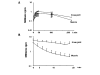
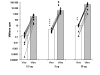
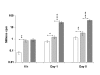
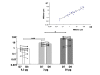
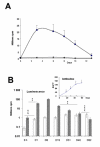
Results: The substrate of luciferase (luciferin) was injected either intraperitonealy (i.p.) or in situ into the muscle or the knee joint. Luminescence resulting from the luciferase-luciferin reaction was measured in vivo with a cooled CCD camera and/or in vitro on tissue lysate. Maximal luminescence of the knee joint and muscle after i.p. (2.5 mg) or local injection of luciferin (50 microg in the knee joint, 100 microg in the muscle) were highly correlated. With the local injection procedure adopted, in vivo and in vitro luminescences measured on the same muscles significantly correlated. Luminescence measurements were reproducible and the signal level was proportional to the amount of plasmid injected. In vivo luciferase activity in the electrotransfered knee joint was detected for two weeks. Intramuscular electrotransfer of 0.3 or 3 microg of plasmid led to stable luciferase expression for 62 days, whereas injecting 30 microg of plasmid resulted in a drop of luminescence three weeks after electrotransfer. These decreases were partially associated with the development of an immune response.
Conclusion: A particular advantage of the i.p. injection of substrate is a widespread distribution at luciferase production sites. We have also highlighted advantages of local injection as a more sensitive detection method with reduced substrate consumption. Besides, this route of injection is relatively free of uncontrolled parameters, such as diffusion to the target organ, crossing of biological barriers and evidencing variations in local enzymatic kinetics, probably related to the reaction medium in the targeted organ. Optical imaging was shown to be a sensitive and relevant technique to quantify variations of luciferase activity in vivo. Further evaluation of the effective amount of luciferase in a given tissue by in vivo optical imaging relies on conditions of the enzymatic reaction and light absorption and presently requires in vitro calibration for each targeted organ.
Figures
Similar articles
-
Electrotransfer of human IL-1Ra into skeletal muscles reduces the incidence of murine collagen-induced arthritis.J Gene Med. 2004 Oct;6(10):1125-33. doi: 10.1002/jgm.599. J Gene Med. 2004. PMID: 15452879
-
Long-term tracing of adenoviral expression in rat and rabbit using luciferase imaging.J Gene Med. 2005 Jun;7(6):792-802. doi: 10.1002/jgm.720. J Gene Med. 2005. PMID: 15712373
-
In vivo monitoring of DNA vaccine gene expression using firefly luciferase as a naked DNA.Vaccine. 2006 Apr 12;24(16):3057-62. doi: 10.1016/j.vaccine.2006.01.033. Epub 2006 Jan 30. Vaccine. 2006. PMID: 16483696
-
Plasmid DNA electrotransfer for intracellular and secreted proteins expression: new methodological developments and applications.J Gene Med. 2004 Feb;6 Suppl 1:S11-23. doi: 10.1002/jgm.508. J Gene Med. 2004. PMID: 14978747 Review.
-
In vivo bioluminescence imaging.Comp Med. 2004 Dec;54(6):631-4. Comp Med. 2004. PMID: 15679260 Review.
Cited by
-
Targeted delivery of proteins into the central nervous system mediated by rabies virus glycoprotein-derived peptide.Pharm Res. 2012 Jun;29(6):1562-9. doi: 10.1007/s11095-012-0667-y. Epub 2012 Jan 10. Pharm Res. 2012. PMID: 22231987
-
Bacterial magnetic particles as a novel and efficient gene vaccine delivery system.Gene Ther. 2012 Dec;19(12):1187-95. doi: 10.1038/gt.2011.197. Epub 2011 Dec 15. Gene Ther. 2012. PMID: 22170341 Free PMC article.
-
Effect of tape stripping and adjuvants on immune response after intradermal DNA electroporation.Pharm Res. 2009 Jul;26(7):1745-51. doi: 10.1007/s11095-009-9885-3. Epub 2009 Apr 21. Pharm Res. 2009. PMID: 19384465
-
Evaluation of immunogen delivery by DNA immunization using non-invasive bioluminescence imaging.Hum Vaccin Immunother. 2013 Oct;9(10):2228-36. doi: 10.4161/hv.25561. Epub 2013 Jul 3. Hum Vaccin Immunother. 2013. PMID: 23896580 Free PMC article.
-
Evaluation of Tumor Cell Response to Hyperthermia with Bioluminescent Imaging.J Basic Clin Med. 2012 Jan 1;1(1):16-19. J Basic Clin Med. 2012. PMID: 23745173 Free PMC article.
References
-
- Harimoto K, Sugimura K, Lee CR, Kuratsukuri K, Kishimoto T. In vivo gene transfer methods in the bladder without viral vectors. Br J Urol. 1998;81:870–874. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

