Lipid rafts in Cryptococcus neoformans concentrate the virulence determinants phospholipase B1 and Cu/Zn superoxide dismutase
- PMID: 16524904
- PMCID: PMC1398056
- DOI: 10.1128/EC.5.3.488-498.2006
Lipid rafts in Cryptococcus neoformans concentrate the virulence determinants phospholipase B1 and Cu/Zn superoxide dismutase
Abstract
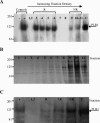
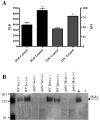
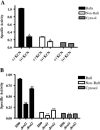
Lipid rafts have been identified in the membranes of mammalian cells, the yeast Saccharomyces cerevisiae, and the pathogenic fungus Candida albicans. Formed by a lateral association of sphingolipids and sterols, rafts concentrate proteins carrying a glycosylphosphatidylinositol (GPI) anchor. We report the isolation of membranes with the characteristics of rafts from the fungal pathogen Cryptococcus neoformans. These characteristics include insolubility in Triton X-100 (TX100) at 4 degrees C, more-buoyant density within a sucrose gradient than the remaining membranes, and threefold enrichment with sterols. The virulence determinant phospholipase B1 (PLB1), a GPI-anchored protein, was highly concentrated in raft membranes and could be displaced from them by treatment with the sterol-sequestering agent methyl-beta-cyclodextrin (MbetaCD). Phospholipase B enzyme activity was inhibited in the raft environment and increased 15-fold following disruption of rafts with TX100 at 37 degrees C. Treatment of viable cryptococcal cells in suspension with MbetaCD also released PLB1 protein and enzyme activity, consistent with localization of PLB1 in plasma membrane rafts prior to secretion. The antioxidant virulence factor Cu/Zn superoxide dismutase (SOD1) was concentrated six- to ninefold in raft membrane fractions compared with nonraft membranes, whereas the cell wall-associated virulence factor laccase was not detected in membranes. We hypothesize that raft membranes function to cluster certain virulence factors at the cell surface to allow efficient access to enzyme substrate and/or to provide rapid release to the external environment.
Figures






Similar articles
-
Cell wall-linked cryptococcal phospholipase B1 is a source of secreted enzyme and a determinant of cell wall integrity.J Biol Chem. 2007 Dec 28;282(52):37508-14. doi: 10.1074/jbc.M707913200. Epub 2007 Oct 18. J Biol Chem. 2007. PMID: 17947228
-
Secretion of cryptococcal phospholipase B1 (PLB1) is regulated by a glycosylphosphatidylinositol (GPI) anchor.Biochem J. 2005 Aug 1;389(Pt 3):803-12. doi: 10.1042/BJ20050063. Biochem J. 2005. PMID: 15826239 Free PMC article.
-
Role of conserved active site residues in catalysis by phospholipase B1 from Cryptococcus neoformans.Biochemistry. 2007 Sep 4;46(35):10024-32. doi: 10.1021/bi7009508. Epub 2007 Aug 9. Biochemistry. 2007. PMID: 17685590
-
Role of laccase in the biology and virulence of Cryptococcus neoformans.FEMS Yeast Res. 2004 Oct;5(1):1-10. doi: 10.1016/j.femsyr.2004.04.004. FEMS Yeast Res. 2004. PMID: 15381117 Review.
-
Interrelationship between the Non-Vesicular Transport of Sterols and Their Distribution between the Rafts and the Non-Raft Phase of the Plasma Membrane.Biochemistry (Mosc). 2025 Mar;90(3):321-333. doi: 10.1134/S0006297924604313. Biochemistry (Mosc). 2025. PMID: 40367076 Review.
Cited by
-
Lipid Secretion by Parasitic Cells of Coccidioides Contributes to Disseminated Disease.Front Cell Infect Microbiol. 2021 May 13;11:592826. doi: 10.3389/fcimb.2021.592826. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34055661 Free PMC article.
-
Comprehensive analysis of glycosylphosphatidylinositol-anchored proteins in Candida albicans.Eukaryot Cell. 2007 Feb;6(2):119-33. doi: 10.1128/EC.00297-06. Epub 2006 Dec 22. Eukaryot Cell. 2007. PMID: 17189485 Free PMC article. Review. No abstract available.
-
Role of sphingolipids in the host-pathogen interaction.Biochim Biophys Acta Mol Cell Biol Lipids. 2023 Nov;1868(11):159384. doi: 10.1016/j.bbalip.2023.159384. Epub 2023 Sep 4. Biochim Biophys Acta Mol Cell Biol Lipids. 2023. PMID: 37673393 Free PMC article.
-
Basidiomycota Fungi and ROS: Genomic Perspective on Key Enzymes Involved in Generation and Mitigation of Reactive Oxygen Species.Front Fungal Biol. 2022 Mar 23;3:837605. doi: 10.3389/ffunb.2022.837605. eCollection 2022. Front Fungal Biol. 2022. PMID: 37746164 Free PMC article. Review.
-
Identifying a novel connection between the fungal plasma membrane and pH-sensing.Mol Microbiol. 2018 Aug;109(4):474-493. doi: 10.1111/mmi.13998. Epub 2018 Sep 9. Mol Microbiol. 2018. PMID: 29885030 Free PMC article.
References
-
- Bourbonnais, R., and M. G. Paice. 1990. Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 267:99-102. - PubMed
-
- Brasitus, T. A., and D. Schachter. 1980. Lipid dynamics and lipid-protein interactions in rat enterocyte basolateral and microvillus membranes. Biochemistry 19:2763-2769. - PubMed
-
- Brouwer, M., T. H. Brouwer, W. Grater, J. J. Enghild, and I. B. Thogersen. 1997. The paradigm that all oxygen-respiring eukaryotes have cytosolic CuZn-superoxide dismutase and that Mn-superoxide dismutase is localized to the mitochondria does not apply to a large group of marine arthropods. Biochemistry 36:13381-13388. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

