Cross talk between a fungal blue-light perception system and the cyclic AMP signaling pathway
- PMID: 16524905
- PMCID: PMC1398060
- DOI: 10.1128/EC.5.3.499-506.2006
Cross talk between a fungal blue-light perception system and the cyclic AMP signaling pathway
Abstract
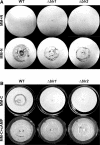
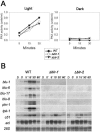
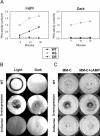
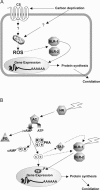
Blue light regulates many physiological and developmental processes in fungi. In Trichoderma atroviride the complex formed by the BLR-1 and BLR-2 proteins appears to play an essential role as a sensor and transcriptional regulator in photoconidiation. Here we demonstrate that the BLR proteins are necessary for carbon deprivation induced conidiation, even in the absence of light, pointing to the existence of an unprecedented cross talk between light and carbon sensing. Further, in contrast to what has been found in all other fungal systems, clear BLR-independent blue-light responses, including the activation of protein kinase A (PKA) and the regulation of gene expression, were found. Expression of an antisense version of the pkr-1 gene, encoding the regulatory subunit of PKA, resulted in a nonsporulating phenotype, whereas overexpression of the gene produced colonies that conidiate even in the dark. In addition, overexpression of pkr-1 blocked the induction of early light response genes. Thus, our data demonstrate that PKA plays an important role in the regulation of light responses in Trichoderma. Together, these observations suggest that the BLR complex plays a general role in sensing environmental cues that trigger conidiation and that such a role can be separated from its function as a transcription factor.
Figures


Similar articles
-
BLR-1 and BLR-2, key regulatory elements of photoconidiation and mycelial growth in Trichoderma atroviride.Microbiology (Reading). 2004 Nov;150(Pt 11):3561-3569. doi: 10.1099/mic.0.27346-0. Microbiology (Reading). 2004. PMID: 15528646
-
Enhanced responsiveness and sensitivity to blue light by blr-2 overexpression in Trichoderma atroviride.Microbiology (Reading). 2007 Nov;153(Pt 11):3909-3922. doi: 10.1099/mic.0.2007/007302-0. Microbiology (Reading). 2007. PMID: 17975098
-
Histone Deacetylase HDA-2 Regulates Trichoderma atroviride Growth, Conidiation, Blue Light Perception, and Oxidative Stress Responses.Appl Environ Microbiol. 2017 Jan 17;83(3):e02922-16. doi: 10.1128/AEM.02922-16. Print 2017 Feb 1. Appl Environ Microbiol. 2017. PMID: 27864177 Free PMC article.
-
Reproduction without sex: conidiation in the filamentous fungus Trichoderma.Microbiology (Reading). 2010 Oct;156(Pt 10):2887-2900. doi: 10.1099/mic.0.041715-0. Epub 2010 Aug 5. Microbiology (Reading). 2010. PMID: 20688823 Review.
-
Trichoderma: sensing the environment for survival and dispersal.Microbiology (Reading). 2012 Jan;158(Pt 1):3-16. doi: 10.1099/mic.0.052688-0. Epub 2011 Sep 29. Microbiology (Reading). 2012. PMID: 21964734 Review.
Cited by
-
Evidence of cAMP involvement in cellobiohydrolase expression and secretion by Trichoderma reesei in presence of the inducer sophorose.BMC Microbiol. 2015 Sep 30;15:195. doi: 10.1186/s12866-015-0536-z. BMC Microbiol. 2015. PMID: 26424592 Free PMC article.
-
Protein kinase A controls yeast growth in visible light.BMC Biol. 2020 Nov 16;18(1):168. doi: 10.1186/s12915-020-00867-4. BMC Biol. 2020. PMID: 33198745 Free PMC article.
-
MAPkinases regulate secondary metabolism, sexual development and light dependent cellulase regulation in Trichoderma reesei.Sci Rep. 2023 Feb 2;13(1):1912. doi: 10.1038/s41598-023-28938-w. Sci Rep. 2023. PMID: 36732590 Free PMC article.
-
The Trichoderma reesei Cry1 protein is a member of the cryptochrome/photolyase family with 6-4 photoproduct repair activity.PLoS One. 2014 Jun 25;9(6):e100625. doi: 10.1371/journal.pone.0100625. eCollection 2014. PLoS One. 2014. PMID: 24964051 Free PMC article.
-
Trichoderma in the light of day--physiology and development.Fungal Genet Biol. 2010 Nov;47(11):909-16. doi: 10.1016/j.fgb.2010.04.010. Epub 2010 May 11. Fungal Genet Biol. 2010. PMID: 20466064 Free PMC article. Review.
References
-
- Arpaia, G., F. Cerri, S. Baima, and G. Macino. 1999. Involvement of protein kinase C in the response of Neurospora crassa to blue light. Mol. Gen. Genet. 262:314-322. - PubMed
-
- Arpaia, G., J. Loros, J. Dunlap, G. Morelli, and G. Macino. 1995. Light induction of the clock-controlled gene ccg-1 is not transduced through the circadian clock in Neurospora crassa. Mol. Gen. Genet. 247:157-163. - PubMed
-
- Baek, J. M., and C. M. Kenerley. 1998. The arg2 gene of Trichoderma virens: cloning and development of a homologous transformation system. Fungal Genet. Biol. 23:34-44. - PubMed
-
- Berrocal-Tito, G., L. Sametz-Baron, K. Eichenberg, B. A. Horwitz, and A. Herrera-Estrella. 1999. Rapid blue light regulation of a Trichoderma harzianum photolyase gene. J. Biol. Chem. 274:14288-14294. - PubMed
-
- Berrocal-Tito, G. M., T. Rosales-Saavedra, A. Herrera-Estrella, and B. A. Horwitz. 2000. Characterization of blue-light and developmental regulation of the photolyase gene phr1 in Trichoderma harzianum. Photochem. Photobiol. 71:662-668. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

