Posttranslational, translational, and transcriptional responses to nitric oxide stress in Cryptococcus neoformans: implications for virulence
- PMID: 16524907
- PMCID: PMC1398057
- DOI: 10.1128/EC.5.3.518-529.2006
Posttranslational, translational, and transcriptional responses to nitric oxide stress in Cryptococcus neoformans: implications for virulence
Abstract
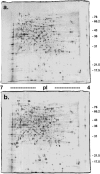
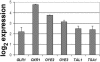
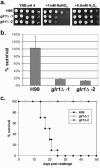
The ability of the fungal pathogen Cryptococcus neoformans to evade the mammalian innate immune response and cause disease is partially due to its ability to respond to and survive nitrosative stress. In this study, we use proteomic and genomic approaches to elucidate the response of C. neoformans to nitric oxide stress. This nitrosative stress response involves both transcriptional, translational, and posttranslational regulation. Proteomic and genomic analyses reveal changes in expression of stress response genes. In addition, genes involved in cell wall organization, respiration, signal transduction, transport, transcriptional control, and metabolism show altered expression under nitrosative conditions. Posttranslational modifications of transaldolase (Tal1), aconitase (Aco1), and the thiol peroxidase, Tsa1, are regulated during nitrosative stress. One stress-related protein up-regulated in the presence of nitric oxide stress is glutathione reductase (Glr1). To further investigate its functional role during nitrosative stress, a deletion mutant was generated. We show that this glr1Delta mutant is sensitive to nitrosative stress and macrophage killing in addition to being avirulent in mice. These studies define the response to nitrosative stress in this important fungal pathogen.
Figures
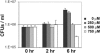
References
-
- Alvarez, B., and R. Radi. 2003. Peroxynitrite reactivity with amino acids and proteins. Amino Acids 25:295-311. - PubMed
-
- Brown, N. M., S. A. Anderson, D. W. Steffen, T. B. Carpenter, M. C. Kennedy, W. E. Walden, and R. S. Eisenstein. 1998. Novel role of phosphorylation in Fe-S cluster stability revealed by phosphomimetic mutations at Ser-138 of iron regulatory protein 1. Proc. Natl. Acad. Sci. USA 95:15235-15240. - PMC - PubMed
-
- Cairo, G., R. Ronchi, S. Recalcati, A. Campanella, and G. Minotti. 2002. Nitric oxide and peroxynitrite activate the iron regulatory protein-1 of J774A.1 macrophages by direct disassembly of the Fe-S cluster of cytoplasmic aconitase. Biochemistry 41:7435-7442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

