The actin gene ACT1 is required for phagocytosis, motility, and cell separation of Tetrahymena thermophila
- PMID: 16524910
- PMCID: PMC1398063
- DOI: 10.1128/EC.5.3.555-567.2006
The actin gene ACT1 is required for phagocytosis, motility, and cell separation of Tetrahymena thermophila
Abstract
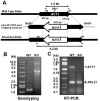
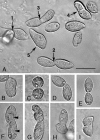
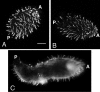
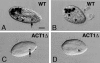
A previously identified Tetrahymena thermophila actin gene (C. G. Cupples and R. E. Pearlman, Proc. Natl. Acad. Sci. USA 83:5160-5164, 1986), here called ACT1, was disrupted by insertion of a neo3 cassette. Cells in which all expressed copies of this gene were disrupted exhibited intermittent and extremely slow motility and severely curtailed phagocytic uptake. Transformation of these cells with inducible genetic constructs that contained a normal ACT1 gene restored motility. Use of an epitope-tagged construct permitted visualization of Act1p in the isolated axonemes of these rescued cells. In ACT1Delta mutant cells, ultrastructural abnormalities of outer doublet microtubules were present in some of the axonemes. Nonetheless, these cells were still able to assemble cilia after deciliation. The nearly paralyzed ACT1Delta cells completed cleavage furrowing normally, but the presumptive daughter cells often failed to separate from one another and later became reintegrated. Clonal analysis revealed that the cell cycle length of the ACT1Delta cells was approximately double that of wild-type controls. Clones could nonetheless be maintained for up to 15 successive fissions, suggesting that the ACT1 gene is not essential for cell viability or growth. Examination of the cell cortex with monoclonal antibodies revealed that whereas elongation of ciliary rows and formation of oral structures were normal, the ciliary rows of reintegrated daughter cells became laterally displaced and sometimes rejoined indiscriminately across the former division furrow. We conclude that Act1p is required in Tetrahymena thermophila primarily for normal ciliary motility and for phagocytosis and secondarily for the final separation of daughter cells.
Figures


Similar articles
-
Analysis of properties of cilia using Tetrahymena thermophila.Methods Mol Biol. 2009;586:283-99. doi: 10.1007/978-1-60761-376-3_16. Methods Mol Biol. 2009. PMID: 19768437
-
Kinesin-II is preferentially targeted to assembling cilia and is required for ciliogenesis and normal cytokinesis in Tetrahymena.Mol Biol Cell. 1999 Oct;10(10):3081-96. doi: 10.1091/mbc.10.10.3081. Mol Biol Cell. 1999. PMID: 10512852 Free PMC article.
-
Strategies for the isolation of ciliary motility and assembly mutants in Tetrahymena.Methods Cell Biol. 1995;47:571-8. doi: 10.1016/s0091-679x(08)60862-6. Methods Cell Biol. 1995. PMID: 7476547 No abstract available.
-
Manipulating ciliary protein-encoding genes in Tetrahymena thermophila.Methods Cell Biol. 2009;93:1-20. doi: 10.1016/S0091-679X(08)93001-6. Epub 2009 Dec 4. Methods Cell Biol. 2009. PMID: 20409809
-
Calmodulin and Ca2+/calmodulin-binding proteins are involved in Tetrahymena thermophila phagocytosis.Cell Struct Funct. 2000 Aug;25(4):243-51. doi: 10.1247/csf.25.243. Cell Struct Funct. 2000. PMID: 11129794
Cited by
-
CCTalpha and CCTdelta chaperonin subunits are essential and required for cilia assembly and maintenance in Tetrahymena.PLoS One. 2010 May 18;5(5):e10704. doi: 10.1371/journal.pone.0010704. PLoS One. 2010. PMID: 20502701 Free PMC article.
-
Ciliate cortical organization and dynamics for cell motility: Comparing ciliates and vertebrates.J Eukaryot Microbiol. 2022 Sep;69(5):e12880. doi: 10.1111/jeu.12880. Epub 2022 Jan 12. J Eukaryot Microbiol. 2022. PMID: 34897878 Free PMC article. Review.
-
Transcriptomic Differences between Free-Living and Parasitic Chilodonella uncinata (Alveolata, Ciliophora).Microorganisms. 2022 Aug 15;10(8):1646. doi: 10.3390/microorganisms10081646. Microorganisms. 2022. PMID: 36014062 Free PMC article.
-
Tetrahymena thermophila: a divergent perspective on membrane traffic.J Exp Zool B Mol Dev Evol. 2014 Nov;322(7):500-16. doi: 10.1002/jez.b.22564. Epub 2014 Mar 14. J Exp Zool B Mol Dev Evol. 2014. PMID: 24634411 Free PMC article. Review.
-
Intracellular connections between basal bodies promote the coordinated behavior of motile cilia.Mol Biol Cell. 2022 Sep 15;33(11):br18. doi: 10.1091/mbc.E22-05-0150. Epub 2022 Jun 29. Mol Biol Cell. 2022. PMID: 35767367 Free PMC article.
References
-
- Bakowska, J., E. M. Nelsen, and J. Frankel. 1982. Development of the ciliary pattern of the oral apparatus of Tetrahymena thermophila. J. Protozool. 29:366-382.
-
- Bré, M. H., V. Redeker, M. Quibell, J. Darmanaden-Delorme, C. Bressac, J. Cosson, P. Huitorel, J. M. Schmitter, J. Rossler, T. Johnson, A. Adoutte, and N. Levilliers. 1996. Axonemal tubulin polyglycylation probed with two monoclonal antibodies: widespread evolutionary distribution, appearance during spermatozoan maturation and possible function in motility. J. Cell Sci. 109:727-738. - PubMed
-
- Brown, J. M., C. Hardin, and J. Gaertig. 1999. Rotokinesis, a novel phenomenon of cell locomotion-assisted cytokinesis in the ciliate Tetrahymena thermophila. Cell Biol. Int. 23:841-848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

