Chemoenzymatic and template-directed synthesis of bioactive macrocyclic peptides
- PMID: 16524919
- PMCID: PMC1393257
- DOI: 10.1128/MMBR.70.1.121-146.2006
Chemoenzymatic and template-directed synthesis of bioactive macrocyclic peptides
Abstract
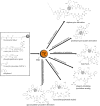
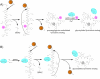
Non-ribosomally synthesized peptides have compelling biological activities ranging from antimicrobial to immunosuppressive and from cytostatic to antitumor. The broad spectrum of applications in modern medicine is reflected in the great structural diversity of these natural products. They contain unique building blocks, such as d-amino acids, fatty acids, sugar moieties, and heterocyclic elements, as well as halogenated, methylated, and formylated residues. In the past decades, significant progress has been made toward the understanding of the biosynthesis of these secondary metabolites by nonribosomal peptide synthetases (NRPSs) and their associated tailoring enzymes. Guided by this knowledge, researchers genetically redesigned the NRPS template to synthesize new peptide products. Moreover, chemoenzymatic strategies were developed to rationally engineer nonribosomal peptides products in order to increase or alter their bioactivities. Specifically, chemical synthesis combined with peptide cyclization mediated by nonribosomal thioesterase domains enabled the synthesis of glycosylated cyclopeptides, inhibitors of integrin receptors, peptide/polyketide hybrids, lipopeptide antibiotics, and streptogramin B antibiotics. In addition to the synthetic potential of these cyclization catalysts, which is the main focus of this review, different enzymes for tailoring of peptide scaffolds as well as the manipulation of carrier proteins with reporter-labeled coenzyme A analogs are discussed.
Figures



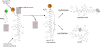
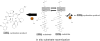


References
-
- Balibar, C. J., F. H. Vaillancourt, and C. T. Walsh. 2005. Generation of d-amino acid residues in assembly of arthrofactin by dual condensation/epimerization domains. Chem. Biol. 12:1189-1200. - PubMed
-
- Ball, L. J., C. M. Goult, J. A. Donarski, J. Micklefield, and V. Ramesh. 2004. NMR structure determination and calcium binding effects of lipopeptide antibiotic daptomycin. Org. Biomol. Chem. 2:1872-1878. - PubMed
-
- Baltz, R. H., P. Brian, V. Miao, and S. K. Wrigley. 2005. Combinatorial biosynthesis of lipopeptide antibiotics in Streptomyces roseosporus. J. Ind. Microbiol. Biotechnol. 2005:1-9. [Online.] - PubMed
-
- Barna, J. C., and D. H. Williams. 1984. The structure and mode of action of glycopeptide antibiotics of the vancomycin group. Annu. Rev. Microbiol. 38:339-357. - PubMed
-
- Beck, Z. Q., C. C. Aldrich, N. A. Magarvey, G. I. Georg, and D. H. Sherman. 2005. Chemoenzymatic synthesis of cryptophycin/arenastatin natural products. Biochemistry 44:13457-13466. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

