Targeting of melanoma brain metastases using engineered neural stem/progenitor cells
- PMID: 16524944
- PMCID: PMC1871940
- DOI: 10.1215/15228517-2005-012
Targeting of melanoma brain metastases using engineered neural stem/progenitor cells
Abstract
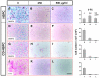
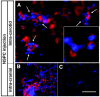
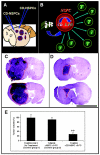
Brain metastases are an increasingly frequent and serious clinical problem for cancer patients, especially those with advanced melanoma. Given the extensive tropism of neural stem/progenitor cells (NSPCs) for pathological areas in the central nervous system, we expanded investigations to determine whether NSPCs could also target multiple sites of brain metastases in a syngeneic experimental melanoma model. Using cytosine deaminase-expressing NSPCs (CD-NSPCs) and systemic 5-fluorocytosine (5-FC) pro-drug administration, we explored their potential as a cell-based targeted drug delivery system to disseminated brain metastases. Our results indicate a strong tropism of NSPCs for intracerebral melanoma metastases. Furthermore, in our therapeutic paradigm, animals with established melanoma brain metastasis received intracranial implantation of CD-NSPCs followed by systemic 5-FC treatment, resulting in a significant (71%) reduction in tumor burden. These data provide proof of principle for the use of NSPCs for targeted delivery of therapeutic gene products to melanoma brain metastases.
Figures
References
-
- Aboody KS, Brown A, Rainov NG, Bower KA, Liu S, Yang W, Small JE, Herrlinger U, Ourednik V, Black PM, Breakefield XO, Snyder EY. Neural stem cells display extensive tropism for pathology in adult brain: Evidence from intracranial gliomas (erratum in Proc. Natl. Acad. Sci. USA [2001] 98, 777). . Proc Natl Acad Sci USA. 2000;97:12846–12851. - PMC - PubMed
-
- Bagnato A, Rosano L, Spinella F, Di Castro V, Tecce R, Natali PG. Endothelin B receptor blockade inhibits dynamics of cell interactions and communications in melanoma cell progression. Cancer Res. 2004;64:1436–1443. - PubMed
-
- Barresi V, Belluardo N, Sipione S, Mudo G, Cattaneo E, Condorelli DF. Transplantation of prodrug-converting neural progenitor cells for brain tumor therapy. Cancer Gene Ther. 2003;10:396–402. - PubMed
-
- Benedetti S, Pirola B, Pollo B, Magrassi L, Bruzzone MG, Rigamonti D, Galli R, Selleri S, Di Meco F, De Fraja C, Vescovi A, Cattaneo E, Finocchiaro G. Gene therapy of experimental brain tumors using neural progenitor cells. Nat Med. 2000;6:447–450. - PubMed
-
- Brown AB, Yang W, Schmidt NO, Carroll R, Leishear KK, Rainov NG, Black PM, Breakefield XO, Aboody KS. Intravascular delivery of neural stem cell lines to target intracranial and extracranial tumors of neural and non-neural origin. Hum Gene Ther. 2003;14:1777–1785. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

