JRAB/MICAL-L2 is a junctional Rab13-binding protein mediating the endocytic recycling of occludin
- PMID: 16525024
- PMCID: PMC1446078
- DOI: 10.1091/mbc.e05-09-0826
JRAB/MICAL-L2 is a junctional Rab13-binding protein mediating the endocytic recycling of occludin
Abstract
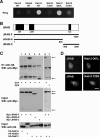
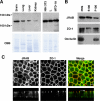
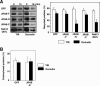
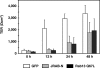
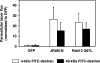
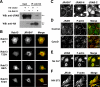
The dynamic turnover of tight junctions (TJs) is essential for epithelial-mesenchymal transitions and/or mesenchymal-epithelial transitions during epithelial morphogenesis. We previously demonstrated that Rab13 specifically mediates the endocytic recycling of occludin. Here, we identified MICAL-L2 (molecule interacting with CasL-like 2) as a novel Rab13-binding protein. Immunoprecipitation and immunofluorescence microscopy showed that MICAL-L2 specifically bound to the GTP-bound form of Rab13 via its C terminus, which contained a coiled-coil domain, and localized at TJs in epithelial MTD-1A cells. Recycling assay demonstrated that a MICAL-L2 mutant lacking the Rab13-binding domain (MICAL-L2-N) specifically inhibited the endocytic recycling of occludin but not transferrin receptor. Ca2+ switch assay further revealed that MICAL-L2-N as well as Rab13 Q67L inhibited the recruitment of occludin to the plasma membrane, the development of transepithelial electrical resistance, and the formation of a paracellular diffusion barrier. MICAL-L2 was displaced from TJs upon actin depolymerization and was distributed along radiating actin cables and stress fibers in Ca2+-depleted MTD-1A and fibroblastic NIH3T3 cells, respectively. These results suggest that MICAL-L2 mediates the endocytic recycling of occludin and the formation of functional TJs by linking Rab13 to actin cytoskeleton. We rename MICAL-L2 as JRAB (junctional Rab13-binding protein).
Figures

Similar articles
-
The interaction of JRAB/MICAL-L2 with Rab8 and Rab13 coordinates the assembly of tight junctions and adherens junctions.Mol Biol Cell. 2008 Mar;19(3):971-83. doi: 10.1091/mbc.e07-06-0551. Epub 2007 Dec 19. Mol Biol Cell. 2008. PMID: 18094055 Free PMC article.
-
Involvement of actinin-4 in the recruitment of JRAB/MICAL-L2 to cell-cell junctions and the formation of functional tight junctions.Mol Cell Biol. 2008 May;28(10):3324-35. doi: 10.1128/MCB.00144-08. Epub 2008 Mar 10. Mol Cell Biol. 2008. PMID: 18332111 Free PMC article.
-
Identification and characterization of JRAB/MICAL-L2, a junctional Rab13-binding protein.Methods Enzymol. 2008;438:141-53. doi: 10.1016/S0076-6879(07)38010-5. Methods Enzymol. 2008. PMID: 18413246
-
Rab family small G proteins in regulation of epithelial apical junctions.Front Biosci (Landmark Ed). 2009 Jan 1;14(6):2115-29. doi: 10.2741/3366. Front Biosci (Landmark Ed). 2009. PMID: 19273188 Review.
-
Regulation of epithelial cell adhesion and repulsion: role of endocytic recycling.J Med Invest. 2008 Feb;55(1-2):9-16. doi: 10.2152/jmi.55.9. J Med Invest. 2008. PMID: 18319540 Review.
Cited by
-
The interaction of JRAB/MICAL-L2 with Rab8 and Rab13 coordinates the assembly of tight junctions and adherens junctions.Mol Biol Cell. 2008 Mar;19(3):971-83. doi: 10.1091/mbc.e07-06-0551. Epub 2007 Dec 19. Mol Biol Cell. 2008. PMID: 18094055 Free PMC article.
-
Important relationships between Rab and MICAL proteins in endocytic trafficking.World J Biol Chem. 2010 Aug 26;1(8):254-64. doi: 10.4331/wjbc.v1.i8.254. World J Biol Chem. 2010. PMID: 21537482 Free PMC article.
-
Caveolae-mediated internalization of occludin and claudin-5 during CCL2-induced tight junction remodeling in brain endothelial cells.J Biol Chem. 2009 Jul 10;284(28):19053-66. doi: 10.1074/jbc.M109.000521. Epub 2009 May 7. J Biol Chem. 2009. PMID: 19423710 Free PMC article.
-
A new locus regulating MICALL2 expression was identified for association with executive inhibition in children with attention deficit hyperactivity disorder.Mol Psychiatry. 2018 Apr;23(4):1014-1020. doi: 10.1038/mp.2017.74. Epub 2017 Apr 18. Mol Psychiatry. 2018. PMID: 28416812
-
Constitutive endocytosis and recycling of NKCC2 in rat thick ascending limbs.Am J Physiol Renal Physiol. 2010 Nov;299(5):F1193-202. doi: 10.1152/ajprenal.00307.2010. Epub 2010 Aug 18. Am J Physiol Renal Physiol. 2010. PMID: 20719977 Free PMC article.
References
-
- Anderson, J. M., Van Itallie, C. M., and Fanning, A. S. (2004). Setting up a selective barrier at the apical junction complex. Curr. Opin. Cell Biol. 16, 140-145. - PubMed
-
- Barrios-Rodiles, M., et al. (2005). High-throughput mapping of a dynamic signaling network in mammalian cells. Science 307, 1621-1625. - PubMed
-
- Bershadsky, A. (2004). Magic touch: how does cell-cell adhesion trigger actin assembly? Trends Cell Biol. 14, 589-593. - PubMed
-
- Bruewer, M., Utech, M., Ivanov, A. I., Hopkins, A. M., Parkos, C. A., and Nusrat, A. (2005). Interferon-γ induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 19, 923-933. - PubMed
-
- D'Atri, F., and Citi, S. (2001). Cingulin interacts with F-actin in vitro. FEBS Lett. 507, 21-24. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

