A novel mechanism of FSH regulation of DNA synthesis in the granulosa cells of hamster preantral follicles: involvement of a protein kinase C-mediated MAP kinase 3/1 self-activation loop
- PMID: 16525034
- PMCID: PMC1482802
- DOI: 10.1095/biolreprod.105.050153
A novel mechanism of FSH regulation of DNA synthesis in the granulosa cells of hamster preantral follicles: involvement of a protein kinase C-mediated MAP kinase 3/1 self-activation loop
Abstract
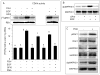
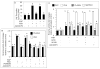
The objective was to reveal whether a protein kinase C (PKC [all isozymes])-mediated self-sustaining MAPK3/1 (3/1 extracellular signal regulated kinase 2/1, also known as ERK2/1) activation loop was necessary for FSH- or epidermal growth factor (EGF)-induced DNA synthesis in the granulosa cells of intact preantral follicles. For this purpose, hamster preantral follicles were cultured with FSH or EGF in the presence of selective kinase inhibitors FSH or EGF phosphorylated RAF1, MAP2K1, and MAPK3/1. However, a relatively higher dose of EGF was necessary to sustain the MAPK3/1 activity, which was essential for cyclin-dependent kinase 4 (CDK4) activation and DNA synthesis. In intact preantral follicles, FSH or EGF stimulated DNA synthesis only in the granulosa cells. Sustained activation of MAPK3/1 beyond 3 h was independent of EGFR kinase activity but dependent on PKC activity, which appeared to form a self-sustaining MAPK3/1 activation loop by activating RAF1, MAP2K1, and PLA2G4 (phospholipase A2 [all cytosolic isozymes]). Inhibition of PKC activity as late as 4 h after the administration of FSH or EGF arrested DNA synthesis, which corresponded with attenuated phosphorylation of RAF1 and MAPK3/1, thus suggesting an essential role of PKC in MAPK3/1 activation. Collectively, these data present a novel self-sustaining mechanism comprised of MAPK3/1, PLA2G4, PKC, and RAF1 for CDK4 activation leading to DNA synthesis in granulosa cells. Either FSH or EGF can activate the loop to activate CDK4 and initiate DNA synthesis; however, consistent with our previous findings, FSH effect seems to be mediated by EGF, which initiates the event by stimulating EGFR kinase.
Figures




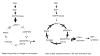
Similar articles
-
Follicle stimulating hormone-induced DNA synthesis in the granulosa cells of hamster preantral follicles involves activation of cyclin-dependent kinase-4 rather than cyclin d2 synthesis.Biol Reprod. 2004 Feb;70(2):509-17. doi: 10.1095/biolreprod.103.023457. Epub 2003 Oct 15. Biol Reprod. 2004. PMID: 14561638
-
Transforming growth factor B1 stimulated DNA synthesis in the granulosa cells of preantral follicles: negative interaction with epidermal growth factor.Biol Reprod. 2006 Jul;75(1):140-8. doi: 10.1095/biolreprod.105.050294. Epub 2006 Mar 8. Biol Reprod. 2006. PMID: 16525033 Free PMC article.
-
Mediation of follicle-stimulating hormone action on follicular deoxyribonucleic acid synthesis by epidermal growth factor.Endocrinology. 1991 Oct;129(4):1903-8. doi: 10.1210/endo-129-4-1903. Endocrinology. 1991. PMID: 1655388
-
The release of EGF domain from EGF-like factors by a specific cleavage enzyme activates the EGFR-MAPK3/1 pathway in both granulosa cells and cumulus cells during the ovulation process.J Reprod Dev. 2012;58(5):510-4. doi: 10.1262/jrd.2012-056. J Reprod Dev. 2012. PMID: 23124701 Review.
-
Regulation of mitogen-activated protein kinase 3/1 activity during meiosis resumption in mammals.J Reprod Dev. 2015;61(6):495-502. doi: 10.1262/jrd.2015-069. J Reprod Dev. 2015. PMID: 26688146 Free PMC article. Review.
Cited by
-
Mapping the follicle-stimulating hormone-induced signaling networks.Front Endocrinol (Lausanne). 2011 Oct 5;2:45. doi: 10.3389/fendo.2011.00045. eCollection 2011. Front Endocrinol (Lausanne). 2011. PMID: 22666216 Free PMC article.
-
Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice.Dev Biol. 2007 May 1;305(1):300-11. doi: 10.1016/j.ydbio.2007.02.019. Epub 2007 Feb 21. Dev Biol. 2007. PMID: 17368609 Free PMC article.
-
Subcellular proteomic approach for identifying the signaling effectors of protein kinase C-β₂ under high glucose conditions in human umbilical vein endothelial cells.Mol Med Rep. 2015 Nov;12(5):7247-62. doi: 10.3892/mmr.2015.4403. Epub 2015 Sep 30. Mol Med Rep. 2015. PMID: 26459836 Free PMC article.
-
Specific protein kinase C isoforms α and βI are involved in follicle-stimulating hormone-induced mouse follicle-enclosed oocytes meiotic resumption.PLoS One. 2012;7(9):e45043. doi: 10.1371/journal.pone.0045043. Epub 2012 Sep 18. PLoS One. 2012. PMID: 23028752 Free PMC article.
-
Molecular regulation of follicle-stimulating hormone synthesis, secretion and action.J Mol Endocrinol. 2018 Apr;60(3):R131-R155. doi: 10.1530/JME-17-0308. Epub 2018 Feb 7. J Mol Endocrinol. 2018. PMID: 29437880 Free PMC article. Review.
References
-
- Yang P, Roy SK. Follicles stimulating hormone-induced DNA synthesis in the granulosa cells of hamster preantral follicles involves activation of cyclin-dependent kinase-4 rather than cyclin D2 synthesis. Biol Reprod. 2004;70:509–517. - PubMed
-
- Cameron MR, Foster JS, Bukovsky A, Wimalasena J. Activation of mitogen-activated protein kinases by gonadotropins and cyclic adenosine 5′- monophosphates in porcine granulosa cells. Biol Reprod. 1996;55:111–119. - PubMed
-
- Roy SK, Greenwald GS. Mediation of follicle-stimulating hormone action on follicular deoxyribonucleic acid synthesis by epidermal growth factor. Endocrinology. 1991;129:1903–1908. - PubMed
-
- Roy SK, Harris SG. Antisense epidermal growth factor oligodeoxynucleotides inhibit follicle-stimulating hormone-induced in vitro DNA and progesterone synthesis in hamster preantral follicles. Mol Endocrinol. 1994;8:1175–1181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

