NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes
- PMID: 16525046
- PMCID: PMC6675155
- DOI: 10.1523/JNEUROSCI.4689-05.2006
NMDA receptors mediate neuron-to-glia signaling in mouse cortical astrocytes
Abstract
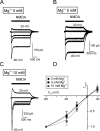
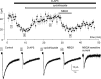
Chemical transmission between neurons and glial cells is an important element of integration in the CNS. Here, we describe currents activated by NMDA in cortical astrocytes, identified in transgenic mice that express enhanced green fluorescent protein under control of the human glial fibrillary acidic protein promoter. Astrocytes were studied by whole-cell voltage clamp either in slices or after gentle nonenzymatic mechanical dissociation. Acutely isolated astrocytes showed a three-component response to glutamate. The initial rapid component was blocked by 1,2,3,4-tetrahydro-6-nitro-2,3-dioxo-benzo[f]quinoxaline-7-sulfonamide (NBQX), which is an antagonist of AMPA receptors (IC50, 2 microM), and the NMDA receptor antagonist D-AP-5 blocked the later sustained component (IC50, 0.6 microM). The third component of glutamate application response was sensitive to D,L-threo-beta-benzyloxyaspartate, a glutamate transporter blocker. Fast application of NMDA evoked concentration-dependent inward currents (EC50, 0.3 microM); these showed use-dependent block by (+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclohepten-5,10-imine maleate (MK-801). These NMDA-evoked currents were linearly dependent on membrane potential and were not affected by extracellular magnesium at concentrations up to 10 mM. Electrical stimulation of axons in layer IV-VI induced a complex inward current in astrocytes situated in the cortical layer II, part of which was sensitive to MK-801 at holding potential -80 mV and was not affected by the AMPA glutamate receptor antagonist NBQX. The fast miniature spontaneous currents were observed in cortical astrocytes in slices as well. These currents exhibited both AMPA and NMDA receptor-mediated components. We conclude that cortical astrocytes express functional NMDA receptors that are devoid of Mg2+ block, and these receptors are involved in neuronal-glial signal transmission.
Figures







References
-
- Akaike N, Moorhouse AJ (2003). Techniques: applications of the nerve-bouton preparation in neuropharmacology. Trends Pharmacol Sci 24:44–47. - PubMed
-
- Akopian G, Kuprijanova E, Kressin K, Steinhuser C (1997). Analysis of ion channel expression by astrocytes in red nucleus brain stem slices of the rat. Glia 19:234–246. - PubMed
-
- Araque A, Carmignoto G, Haydon PG (2001). Dynamic signaling between astrocytes and neurons. Annu Rev Physiol 63:795–813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
