Glial cell line-derived neurotrophic factor-dependent recruitment of Ret into lipid rafts enhances signaling by partitioning Ret from proteasome-dependent degradation
- PMID: 16525057
- PMCID: PMC6675173
- DOI: 10.1523/JNEUROSCI.3420-05.2006
Glial cell line-derived neurotrophic factor-dependent recruitment of Ret into lipid rafts enhances signaling by partitioning Ret from proteasome-dependent degradation
Abstract
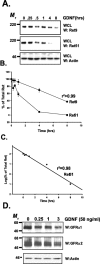
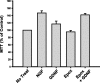
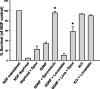
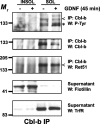
The receptor tyrosine kinase (RTK) Ret is activated by the formation of a complex consisting of ligands such as glial cell line-derived neurotrophic factor (GDNF) and glycerophosphatidylinositol-anchored coreceptors termed GFRalphas. During activation, Ret translocates into lipid rafts, which is critical for functional responses to GDNF. We found that Ret was rapidly ubiquitinated and degraded in sympathetic neurons when activated with GDNF, but, unlike other RTKs that are trafficked to lysosomes for degradation, Ret was degraded predominantly by the proteasome. After GDNF stimulation, the majority of ubiquitinated Ret was located outside of lipid rafts and Ret was lost predominantly from nonraft membrane domains. Consistent with the predominance of Ret degradation outside of rafts, disruption of lipid rafts in neurons did not alter either the GDNF-dependent ubiquitination or degradation of Ret. GDNF-mediated survival of sympathetic neurons was inhibited by lipid raft depletion, and this inhibitory effect of raft disruption on GDNF-mediated survival was reversed if Ret degradation was blocked via proteasome inhibition. Therefore, lipid rafts sequester Ret away from the degradation machinery located in nonraft membrane domains, such as Cbl family E3 ligases, thereby sustaining Ret signaling.
Figures




Similar articles
-
Lipid Rafts Are Physiologic Membrane Microdomains Necessary for the Morphogenic and Developmental Functions of Glial Cell Line-Derived Neurotrophic Factor In Vivo.J Neurosci. 2015 Sep 23;35(38):13233-43. doi: 10.1523/JNEUROSCI.2935-14.2015. J Neurosci. 2015. PMID: 26400951 Free PMC article.
-
CD2AP and Cbl-3/Cbl-c constitute a critical checkpoint in the regulation of ret signal transduction.J Neurosci. 2008 Aug 27;28(35):8789-800. doi: 10.1523/JNEUROSCI.2738-08.2008. J Neurosci. 2008. PMID: 18753381 Free PMC article.
-
c-Src is required for glial cell line-derived neurotrophic factor (GDNF) family ligand-mediated neuronal survival via a phosphatidylinositol-3 kinase (PI-3K)-dependent pathway.J Neurosci. 2001 Mar 1;21(5):1464-72. doi: 10.1523/JNEUROSCI.21-05-01464.2001. J Neurosci. 2001. PMID: 11222636 Free PMC article.
-
GDNF recruits the signaling crew into lipid rafts.Trends Neurosci. 2001 Aug;24(8):427-9. doi: 10.1016/s0166-2236(00)01864-6. Trends Neurosci. 2001. PMID: 11476867 Review.
-
Lipid Raft-Excluded GFRα1/Ret Fails to Transmit GDNF Signaling In Vivo.J Neurosci. 2016 Feb 3;36(5):1442-4. doi: 10.1523/JNEUROSCI.4155-15.2016. J Neurosci. 2016. PMID: 26843628 Free PMC article. Review. No abstract available.
Cited by
-
7-dehydrocholesterol efficiently supports Ret signaling in a mouse model of Smith-Opitz-Lemli syndrome.Sci Rep. 2016 Jun 23;6:28534. doi: 10.1038/srep28534. Sci Rep. 2016. PMID: 27334845 Free PMC article.
-
Gene-environment interactions and the enteric nervous system: Neural plasticity and Hirschsprung disease prevention.Dev Biol. 2016 Sep 15;417(2):188-97. doi: 10.1016/j.ydbio.2016.03.017. Epub 2016 Mar 17. Dev Biol. 2016. PMID: 26997034 Free PMC article. Review.
-
Alternative splicing results in RET isoforms with distinct trafficking properties.Mol Biol Cell. 2012 Oct;23(19):3838-50. doi: 10.1091/mbc.E12-02-0114. Epub 2012 Aug 8. Mol Biol Cell. 2012. PMID: 22875993 Free PMC article.
-
Brain ischemia downregulates the neuroprotective GDNF-Ret signaling by a calpain-dependent mechanism in cultured hippocampal neurons.Cell Death Dis. 2015 Feb 12;6(2):e1645. doi: 10.1038/cddis.2014.578. Cell Death Dis. 2015. PMID: 25675305 Free PMC article.
-
Manganese-Mediated Decrease in Levels of c-RET and Tyrosine Hydroxylase Expression In Vitro.Neurotox Res. 2017 Nov;32(4):661-670. doi: 10.1007/s12640-017-9783-0. Epub 2017 Jul 20. Neurotox Res. 2017. PMID: 28730349
References
-
- Aguilar RC, Wendland B (2003). Ubiquitin: not just for proteasomes anymore. Curr Opin Cell Biol 15:184–190. - PubMed
-
- Airaksinen MS, Saarma M (2002). The GDNF family: signalling, biological functions and therapeutic value. Nat Rev 3:383–394. - PubMed
-
- Anderson RG (1998). The caveolae membrane system. Annu Rev Biochem 67:199–225. - PubMed
-
- Arenas E, Trupp M, Akerud P, Ibanez CF (1995). GDNF prevents degeneration and promotes the phenotype of brain noradrenergic neurons in vivo. Neuron 15:1465–1473. - PubMed
-
- Baloh RH, Enomoto H, Johnson EM Jr, Milbrandt J (2000). The GDNF family ligands and receptors-implications for neural development. Curr Opin Neurobiol 10:103–110. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
