Differential contributions of Caenorhabditis elegans histone deacetylases to huntingtin polyglutamine toxicity
- PMID: 16525063
- PMCID: PMC6675170
- DOI: 10.1523/JNEUROSCI.3344-05.2006
Differential contributions of Caenorhabditis elegans histone deacetylases to huntingtin polyglutamine toxicity
Abstract
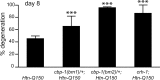
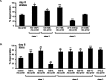
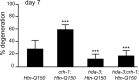
Expansion of a polyglutamine tract in the huntingtin protein causes neuronal degeneration and death in Huntington's disease patients, but the molecular mechanisms underlying polyglutamine-mediated cell death remain unclear. Previous studies suggest that expanded polyglutamine tracts alter transcription by sequestering glutamine rich transcriptional regulatory proteins, thereby perturbing their function. We tested this hypothesis in Caenorhabditis elegans neurons expressing a human huntingtin fragment with an expanded polyglutamine tract (Htn-Q150). Loss of function alleles and RNA interference (RNAi) were used to examine contributions of C. elegans cAMP response element-binding protein (CREB), CREB binding protein (CBP), and histone deacetylases (HDACs) to polyglutamine-induced neurodegeneration. Deletion of CREB (crh-1) or loss of one copy of CBP (cbp-1) enhanced polyglutamine toxicity in C. elegans neurons. Loss of function alleles and RNAi were then used to systematically reduce function of each C. elegans HDAC. Generally, knockdown of individual C. elegans HDACs enhanced Htn-Q150 toxicity, but knockdown of C. elegans hda-3 suppressed toxicity. Neuronal expression of hda-3 restored Htn-Q150 toxicity and suggested that C. elegans HDAC3 (HDA-3) acts within neurons to promote degeneration in response to Htn-Q150. Genetic epistasis experiments suggested that HDA-3 and CRH-1 (C. elegans CREB homolog) directly oppose each other in regulating transcription of genes involved in polyglutamine toxicity. hda-3 loss of function failed to suppress increased neurodegeneration in hda-1/+;Htn-Q150 animals, indicating that HDA-1 and HDA-3 have different targets with opposing effects on polyglutamine toxicity. Our results suggest that polyglutamine expansions perturb transcription of CREB/CBP targets and that specific targeting of HDACs will be useful in reducing associated neurodegeneration.
Figures
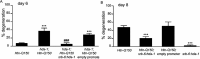

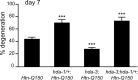
References
-
- Ajamian F, Salminen A, Reeben M (2004). Selective regulation of class I and class II histone deacetylases expression by inhibitors of histone deacetylases in cultured mouse neural cells. Neurosci Lett 365:64–68. - PubMed
-
- Baran R, Aronoff R, Garriga G (1999). The C. elegans homeodomain gene unc-42 regulates chemosensory and glutamate receptor expression. Development 126:2241–2251. - PubMed
-
- Boutell JM, Thomas P, Neal JW, Weston VJ, Duce J, Harper PS, Jones AL (1999). Aberrant interactions of transcriptional repressor proteins with the Huntington's disease gene product, huntingtin. Hum Mol Genet 8:1647–1655. - PubMed
-
- Brachmann CB, Sherman JM, Devine SE, Cameron EE, Pillus L, Boeke JD (1995). The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev 9:2888–2902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
