Diagnosing infection levels of four human malaria parasite species by a polymerase chain reaction/ligase detection reaction fluorescent microsphere-based assay
- PMID: 16525099
- PMCID: PMC3728833
Diagnosing infection levels of four human malaria parasite species by a polymerase chain reaction/ligase detection reaction fluorescent microsphere-based assay
Abstract
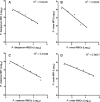
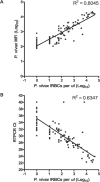
Improving strategies for diagnosing infection by the four human Plasmodium species parasites is important as field-based epidemiologic and clinical studies focused on malaria become more ambitious. Expectations for malaria diagnostic assays include rapid processing with minimal expertise, very high specificity and sensitivity, and quantitative evaluation of parasitemia to be delivered at a very low cost. Toward fulfilling many of these expectations, we have developed a post-polymerase chain reaction (PCR)/ligase detection reaction-fluorescent microsphere assay (LDR-FMA). This assay, which uses Luminex FlexMAP microspheres, provides simultaneous, semi-quantitative detection of infection by all four human malaria parasite species at a sensitivity and specificity equal to other PCR-based assays. In blinded studies using P. falciparum-infected blood from in vitro cultures, we identified infected and uninfected samples with 100% concordance. Additionally, in analyses of P. falciparum in vitro cultures and P. vivax-infected monkeys, comparisons between parasitemia and LDR-FMA signal intensity showed very strong positive correlations (r > 0.95). Application of this multiplex Plasmodium species LDR-FMA diagnostic assay will increase the speed, accuracy, and reliability of diagnosing human Plasmodium species infections in epidemiologic studies of complex malaria-endemic settings.
Figures

References
-
- Russell PF. Man’s Mastery of Malaria. London: Oxford University Press; 1955.
-
- Bruce-Chwatt LJ. Essential Malariology. London: William Heinemann Medical Books; 1985.
-
- Richie TL, Saul A. Progress and challenges for malaria vaccines. Nature. 2002;415:694–701. - PubMed
-
- Greenwood B. The use of anti-malarial drugs to prevent malaria in the population of malaria-endemic areas. Am J Trop Med Hyg. 2004;70:1–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
