An arginine/lysine-rich motif is crucial for VCP/p97-mediated modulation of ataxin-3 fibrillogenesis
- PMID: 16525503
- PMCID: PMC1440312
- DOI: 10.1038/sj.emboj.7601043
An arginine/lysine-rich motif is crucial for VCP/p97-mediated modulation of ataxin-3 fibrillogenesis
Abstract
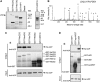
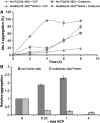
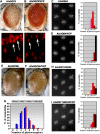
Arginine/lysine-rich motifs typically function as targeting signals for the translocation of proteins to the nucleus. Here, we demonstrate that such a motif consisting of four basic amino acids in the polyglutamine protein ataxin-3 (Atx-3) serves as a recognition site for the interaction with the molecular chaperone VCP. Through this interaction, VCP modulates the fibrillogenesis of pathogenic forms of Atx-3 in a concentration-dependent manner, with low concentrations of VCP stimulating fibrillogenesis and excess concentrations suppressing it. No such effect was observed with a mutant Atx-3 variant, which does not contain a functional VCP interaction motif. Strikingly, a stretch of four basic amino acids in the ubiquitin chain assembly factor E4B was also discovered to be critical for VCP binding, indicating that arginine/lysine-rich motifs might be generally utilized by VCP for the targeting of proteins. In vivo studies with Drosophila models confirmed that VCP selectively modulates aggregation and neurotoxicity induced by pathogenic Atx-3. Together, these results define the VCP-Atx-3 association as a potential target for therapeutic intervention and suggest that it might influence the progression of spinocerebellar ataxia type 3.
Figures



References
-
- Albrecht M, Golatta M, Wullner U, Lengauer T (2004) Structural and functional analysis of ataxin-2 and ataxin-3. Eur J Biochem 271: 3155–3170 - PubMed
-
- Burnett B, Li F, Pittman RN (2003) The polyglutamine neurodegenerative protein ataxin-3 binds polyubiquitylated proteins and has ubiquitin protease activity. Hum Mol Genet 12: 3195–3205 - PubMed
-
- Chai Y, Koppenhafer SL, Shoesmith SJ, Perez MK, Paulson HL (1999) Evidence for proteasome involvement in polyglutamine disease: localization to nuclear inclusions in SCA3/MJD and suppression of polyglutamine aggregation in vitro. Hum Mol Genet 8: 673–682 - PubMed
-
- Chernoff YO, Lindquist SL, Ono B, Inge-Vechtomov SG, Liebman SW (1995) Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+]. Science 268: 880–884 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

