Dynamic behaviour of half-sarcomeres during and after stretch in activated rabbit psoas myofibrils: sarcomere asymmetry but no 'sarcomere popping'
- PMID: 16527855
- PMCID: PMC1618761
- DOI: 10.1113/jphysiol.2006.105809
Dynamic behaviour of half-sarcomeres during and after stretch in activated rabbit psoas myofibrils: sarcomere asymmetry but no 'sarcomere popping'
Abstract
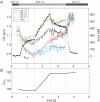
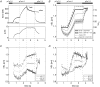
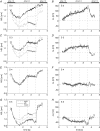
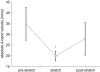
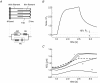
We examined length changes of individual half-sarcomeres during and after stretch in actively contracting, single rabbit psoas myofibrils containing 10-30 sarcomeres. The myofibrils were fluorescently immunostained so that both Z-lines and M-bands of sarcomeres could be monitored by video microscopy simultaneously with the force measurement. Half-sarcomere lengths were determined by processing of video images and tracking the fluorescent Z-line and M-band signals. Upon Ca2+ activation, during the rise in force, active half-sarcomeres predominantly shorten but to different extents so that an active myofibril consists of half-sarcomeres of different lengths and thus asymmetric sarcomeres, i.e. shifted A-bands, indicating different amounts of filament overlap in the two halves. When force reached a plateau, the myofibril was stretched by 15-20% resting length (L0) at a velocity of approximately 0.2 L0 s(-1). The myofibril force response to a ramp stretch is similar to that reported from muscle fibres. Despite the approximately 2.5-fold increase in force due to the stretch, the variability in half-sarcomere length remained almost constant during the stretch and A-band shifts did not progress further, independent of whether half-sarcomeres shortened or lengthened during the initial Ca2+ activation. Moreover, albeit half-sarcomeres lengthened to different extents during a stretch, rapid elongation of individual sarcomeres beyond filament overlap ('popping') was not observed. Thus, in contrast to predictions of the 'popping sarcomere' hypothesis, a stretch rather stabilizes the uniformity of half-sarcomere lengths and sarcomere symmetry. In general, the half-sarcomere length changes (dynamics) before and after stretch were slow and the dynamics after stretch were not readily predictable on the basis of the steady-state force-sarcomere length relation.
Figures



Comment in
-
Why stretched muscles hurt--is there a role for half-sarcomere dynamics?J Physiol. 2006 May 15;573(Pt 1):4. doi: 10.1113/jphysiol.2006.109918. Epub 2006 Mar 31. J Physiol. 2006. PMID: 16581854 Free PMC article. Review. No abstract available.
-
Sarcomere popping requires stretch over a range where total tension decreases with length.J Physiol. 2006 Jul 15;574(Pt 2):627-8; author reply 629-30. doi: 10.1113/jphysiol.2006.574201. J Physiol. 2006. PMID: 16844791 Free PMC article. No abstract available.
References
-
- Abbott BC, Aubert XM. Changes of energy in a muscle during very slow stretches. Proc R Soc Lond B Biol Sci. 1951;139:104–117. - PubMed
-
- Allinger TL, Epstein M, Herzog W. Stability of muscle fibers on the descending limb of the force-length relation. A theoretical consideration. J Biomech. 1996;29:627–633. - PubMed
-
- Brown LM, Hill L. Some observations on variations in filament overlap in tetanized muscle fibres and fibres stretched during a tetanus, detected in the electron microscope after rapid fixation. J Muscle Res Cell Motil. 1991;12:171–182. - PubMed
-
- Curtin NA, Davis RE. Chemical and mechanical changes during stretching of activated frog skeletal muscle. Cold Spring Harb Symp Quant Biol. 1973;37:619–626.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

