S-nitrosothiol signaling in respiratory biology
- PMID: 16528016
- PMCID: PMC2662966
- DOI: 10.1164/rccm.200510-1584PP
S-nitrosothiol signaling in respiratory biology
Abstract
Genetic and biochemical data demonstrate a pivotal role for S-nitrosothiols (SNOs) in mediating the actions of nitric oxide synthases (NOSs). SNOs serve to convey NO bioactivity and to regulate protein function. This understanding is of immediate interest to the pulmonary clinical and research communities. This article reviews the following: (1) biochemical and cellular evidence that SNOs in amino acids, peptides, and proteins elicit NOS-dependent signaling in the respiratory system and (2) studies that link SNO signaling to pulmonary medicine. SNO-mediated signaling is involved in the regulation of minute ventilation, ventilation-perfusion matching, pulmonary arterial pressure, basal airway tone, and respiratory and peripheral muscle function. Derangements in SNO signaling are implicated in many disorders relevant to pulmonary and critical care medicine, including apnea, hypoxemia, pulmonary hypertension, asthma, cystic fibrosis, pneumonia, and septic shock.
Figures


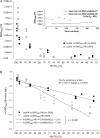

References
-
- Liu L, Hausladen A, Zeng M, Que L, Heitman J, Stamler JS. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature 2001;410:490–494. - PubMed
-
- Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T, Marshall HE, Que LG, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell 2004;116:617–628. - PubMed
-
- Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat Rev Mol Cell Biol 2005;6:150–166. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

