Core protein cleavage by signal peptide peptidase is required for hepatitis C virus-like particle assembly
- PMID: 16528035
- PMCID: PMC2220033
- DOI: 10.1099/vir.0.81664-0
Core protein cleavage by signal peptide peptidase is required for hepatitis C virus-like particle assembly
Abstract
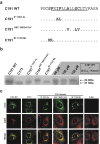
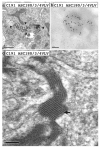
Hepatitis C virus (HCV) core protein, expressed with a Semliki Forest virus replicon, self-assembles into HCV-like particles (HCV-LP) at the endoplasmic reticulum (ER) membrane, providing an opportunity to study HCV assembly and morphogenesis by electron microscopy. This model was used to investigate whether the processing of the HCV core protein by the signal peptide peptidase (SPP) is required for the HCV-LP assembly. Several mutants were designed as there are conflicting reports concerning the cleavage of mutant proteins by SPP. Production of the only core mutant protein that escaped SPP processing led to the formation of multiple layers of electron-dense ER membrane, with no evidence of HCV-LP assembly. These data shed light on the HCV core residues involved in SPP cleavage and suggest that this cleavage is essential for HCV assembly.
Figures

References
-
- Aizaki H, Nagamori S, Matsuda M, et al. Production and release of infectious hepatitis C virus from human liver cell cultures in the three-dimensional radial-flow bioreactor. Virology. 2003;314:16–25. - PubMed
-
- Edbauer D, Winkler E, Regula JT, Pesold B, Steiner H, Haass C. Reconstitution of gamma-secretase activity. Nat Cell Biol. 2003;5:486–488. - PubMed
-
- Esposito G, Scarselli E, Cerino A, Mondelli MU, La Monica N, Traboni C. A human antibody specific for hepatitis C virus core protein: synthesis in a bacterial system and characterization. Gene. 1995;164:203–209. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

