Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of Claudin-16
- PMID: 16528408
- PMCID: PMC1395478
- DOI: 10.1172/JCI26323
Disease-associated mutations affect intracellular traffic and paracellular Mg2+ transport function of Claudin-16
Abstract
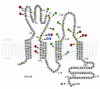
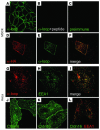
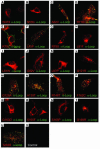
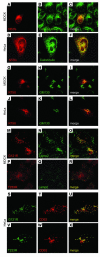
Claudin-16 (Cldn16) is selectively expressed at tight junctions (TJs) of renal epithelial cells of the thick ascending limb of Henle's loop, where it plays a central role in the reabsorption of divalent cations. Over 20 different mutations in the CLDN16 gene have been identified in patients with familial hypomagnesemia with hypercalciuria and nephrocalcinosis (FHHNC), a disease of excessive renal Mg2+ and Ca2+ excretion. Here we show that disease-causing mutations can lead to the intracellular retention of Cldn16 or affect its capacity to facilitate paracellular Mg2+ transport. Nine of the 21 Cldn16 mutants we characterized were retained in the endoplasmic reticulum, where they underwent proteasomal degradation. Three mutants accumulated in the Golgi complex. Two mutants were efficiently delivered to lysosomes, one via clathrin-mediated endocytosis following transport to the cell surface and the other without appearing on the plasma membrane. The remaining 7 mutants localized to TJs, and 4 were found to be defective in paracellular Mg2+ transport. We demonstrate that pharmacological chaperones rescued surface expression of several retained Cldn16 mutants. We conclude that FHHNC can result from mutations in Cldn16 that affect intracellular trafficking or paracellular Mg2+ permeability. Knowledge of the molecular defects associated with disease-causing Cldn16 mutations may open new venues for therapeutic intervention.
Figures
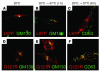
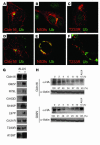
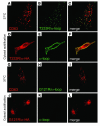
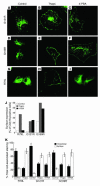

References
-
- Asplin J.R., Favus M.J., Coe F.L. In The kidney. 5th edition. B.M. Brenner, editor. W.B. Saunders.; Philadelphia, Pennsylvania, USA.: 1996. Nephrolithiasis. pp. 1893–1935.
-
- Weber S., et al. Familial hypomagnesemia with hypercalciuria and nephrocalcinosis maps to chromosome 3q27 and is associated with mutations in the PCLN-1 gene. Eur. J. Hum. Genet. 2000;8:414–422. - PubMed
-
- Blanchard A., et al. Paracellin-1 is critical for magnesium and calcium reabsorption in the human thick ascending limb of Henle. Kidney Int. 2001;59:2206–2215. - PubMed
-
- Simon D.B., et al. Paracellin-1, a renal tight junction protein required for paracellular Mg2+ resorption. Science. 1999;285:103–106. - PubMed
-
- Weber S., et al. Primary gene structure and expression studies of rodent paracellin-1. J. Am. Soc. Nephrol. 2001;12:2664–2672. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

