Bone marrow stromal cells induce BMP2/4 production in oxygen-glucose-deprived astrocytes, which promotes an astrocytic phenotype in adult subventricular progenitor cells
- PMID: 16528751
- PMCID: PMC3106272
- DOI: 10.1002/jnr.20834
Bone marrow stromal cells induce BMP2/4 production in oxygen-glucose-deprived astrocytes, which promotes an astrocytic phenotype in adult subventricular progenitor cells
Abstract
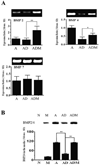
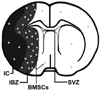
Bone morphogenetic proteins (BMPs) affect cell proliferation and differentiation. Astrocytes in ischemic brain are highly responsive to bone marrow stromal cell (BMSC) treatment. We investigated the effects of BMSCs on astrocytes cultured under oxygen- and glucose-deprived conditions, which in part simulate in vivo stroke conditions, to test the hypothesis that BMSCs alter astrocytic expression of BMPs which may contribute to neurological functional recovery of stroke. Quantitative real-time RT-PCR showed that the expression of BMP2/4 mRNAs decreased within ischemic astrocytes, In contrast, BMP2/4 mRNA was significantly increased after cocultured with BMSCs. Western blotting also confirmed this increase at the protein level in the medium of ischemic astrocytes after coculture with BMSCs. As a source of neural stem and progenitor cells, cultured subventricular zone (SVZ) neurospheres exposed to medium obtained from ischemic astrocytes cocultured with BMSCs were significantly enriched in cells expressing the astrocytic marker glial fibrillary acidic protein (GFAP), but not at the expense of beta-III-tubulin-positive SVZ neuroblasts. The expression of BMP2/4 subsequently increased the phosphorylation of downstream effector Smad1 and the expression of notch signal pathway-induced protein Hes1 in cultured SVZ neurospheres. BMP antagonist Noggin blocked the elevation of phosphorylated Smad1 and the expression of Hes1 as well as reducing the percentage of astrocytic SVZ progenitor cells. Our results indicate that BMSCs increase BMP2/4 expression in ischemic astrocytes. These changes enhance subventricular progenitor cell gliogenesis by activating relevant signaling pathways. BMSC-stimulated signaling of endogenous astrocytes may alter the ischemic environment, promoting remodeling of brain and hence, improve functional recovery after stroke.
(c) 2006 Wiley-Liss, Inc.
Figures


References
-
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. - PubMed
-
- Chalazonitis A, D’Autreaux F, Guha U, Pham TD, Faure C, Chen JJ, Roman D, Kan L, Rothman TP, Kessler JA, Gershon MD. Bone morphogenetic protein-2 and -4 limit the number of enteric neurons but promote development of a TrkC-expressing neurotrophin-3-dependent subset. J Neurosci. 2004;24:4266–4282. - PMC - PubMed
-
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–1011. - PubMed
-
- Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003a;73:778–786. - PubMed
-
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003b;92:692–699. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

