Effect of valdecoxib pretreatment on pain and secondary hyperalgesia: a randomized controlled trial in healthy volunteers [ISRCTN05282752, NCT00260325]
- PMID: 16529650
- PMCID: PMC1420273
- DOI: 10.1186/1471-2253-6-3
Effect of valdecoxib pretreatment on pain and secondary hyperalgesia: a randomized controlled trial in healthy volunteers [ISRCTN05282752, NCT00260325]
Abstract
Background: Induction of the COX-2 isoenzyme appears to play a major role in the genesis of central sensitization after nociceptive stimulation. This study aimed to investigate the efficacy of a single, oral dose of the specific COX-2 inhibitor-valdecoxib in attenuating the central sensitization - induced secondary hyperalgesia in a heat/capsaicin pain model in healthy volunteers.
Methods: The study was a randomized, double blind, placebo controlled, crossover, single dose efficacy trial using 20 healthy volunteers. Two hours following placebo or 40 mg, PO valdecoxib, participants underwent skin sensitization with heat/capsaicin, as well as supra-threshold pain and re-kindling measurements according to an established, validated pain model. Subjects rated pain intensity and unpleasantness on a visual analog scale and the area of secondary hyperalgesia was serially mapped.
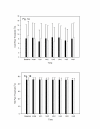
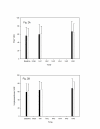
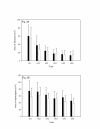
Results: The area of secondary hyperalgesia produced after 40 mg of valdecoxib was no different than that after placebo. Furthermore, there were no significantly relevant differences when volunteers were treated with valdecoxib or placebo in relation to either cold- or hot pain threshold or the intensity of pain after supra-threshold, thermal pain stimulation.
Conclusion: We demonstrated that a single, oral dose of valdecoxib when does not attenuate secondary hyperalgesia induced by heat/capsaicin in a cutaneous sensitization pain model in healthy volunteers.
Figures
Similar articles
-
Effect of systemic adenosine on pain and secondary hyperalgesia associated with the heat/capsaicin sensitization model in healthy volunteers.Reg Anesth Pain Med. 2001 Sep-Oct;26(5):414-9. doi: 10.1053/rapm.2001.22256a. Reg Anesth Pain Med. 2001. PMID: 11561260 Clinical Trial.
-
Is heat pain detection threshold associated with the area of secondary hyperalgesia following brief thermal sensitization? A study of healthy volunteers - design and detailed plan of analysis.BMC Anesthesiol. 2016 May 31;16(1):28. doi: 10.1186/s12871-016-0193-2. BMC Anesthesiol. 2016. PMID: 27246322 Free PMC article. Clinical Trial.
-
Gender differences in pain and secondary hyperalgesia after heat/capsaicin sensitization in healthy volunteers.J Pain. 2006 Mar;7(3):211-7. doi: 10.1016/j.jpain.2005.10.013. J Pain. 2006. PMID: 16516827
-
The effect of psychological manipulations on the development of secondary hyperalgesia: a critical review.Pain Rep. 2025 May 27;10(4):e1291. doi: 10.1097/PR9.0000000000001291. eCollection 2025 Aug. Pain Rep. 2025. PMID: 40444022 Free PMC article. Review.
-
Sensitization of supra-threshold pain responses-Translational aspects and mechanisms.Front Netw Physiol. 2022 Dec 16;2:1078890. doi: 10.3389/fnetp.2022.1078890. eCollection 2022. Front Netw Physiol. 2022. PMID: 36926107 Free PMC article. Review.
Cited by
-
Constitutive cyclooxygenase-2 is involved in central nociceptive processes in humans.Anesthesiology. 2007 May;106(5):1013-8. doi: 10.1097/01.anes.0000265162.39932.33. Anesthesiology. 2007. PMID: 17457134 Free PMC article. Clinical Trial.
-
Does Electroacupuncture Have Different Effects on Peripheral and Central Sensitization in Humans: A Randomized Controlled Study.Front Integr Neurosci. 2019 Oct 15;13:61. doi: 10.3389/fnint.2019.00061. eCollection 2019. Front Integr Neurosci. 2019. PMID: 31680888 Free PMC article.
-
A literature review on the pharmacological sensitivity of human evoked hyperalgesia pain models.Br J Clin Pharmacol. 2016 Oct;82(4):903-22. doi: 10.1111/bcp.13018. Epub 2016 Jul 8. Br J Clin Pharmacol. 2016. PMID: 27203797 Free PMC article. Review.
-
Central sensitization: implications for the diagnosis and treatment of pain.Pain. 2011 Mar;152(3 Suppl):S2-S15. doi: 10.1016/j.pain.2010.09.030. Epub 2010 Oct 18. Pain. 2011. PMID: 20961685 Free PMC article. Review.
-
Distinct BOLD fMRI Responses of Capsaicin-Induced Thermal Sensation Reveal Pain-Related Brain Activation in Nonhuman Primates.PLoS One. 2016 Jun 16;11(6):e0156805. doi: 10.1371/journal.pone.0156805. eCollection 2016. PLoS One. 2016. PMID: 27309348 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

