An effective cancer vaccine modality: lentiviral modification of dendritic cells expressing multiple cancer-specific antigens
- PMID: 16530303
- PMCID: PMC1850619
- DOI: 10.1016/j.vaccine.2006.02.025
An effective cancer vaccine modality: lentiviral modification of dendritic cells expressing multiple cancer-specific antigens
Abstract
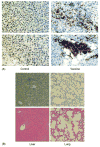
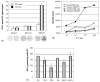
Viral modification of dendritic cells (DCs) may deliver a "danger signal" critical to the hypo-reactive DCs in cancer patients. Using three highly differentially expressed hepatoma tumor-associated antigens (TAAs): stem cell antigen-2 (Sca-2), glycoprotein 38 (GP38) and cellular retinoic acid binding protein 1 (RABP1), we explored the therapeutic potential of the DCs modified with lentiviral vectors (LVs). Preventive and therapeutic injection of the LV-TAA-DC vaccine into tumor-bearing mice elicited a strong anti-tumor response and extended survival, which was associated with tumor-specific interferon-gamma and cytotoxic T cell responses. In vivo elimination of the LV-TAA-DCs by a co-expressed thymidine kinase suicide gene abrogated the therapeutic effect. The modification of DCs with LVs encoding multiple TAAs offers a great opportunity in cancer immunotherapy.
Figures






Similar articles
-
The boosting effect of co-transduction with cytokine genes on cancer vaccine therapy using genetically modified dendritic cells expressing tumor-associated antigen.Int J Oncol. 2006 Apr;28(4):947-53. Int J Oncol. 2006. PMID: 16525645
-
NKT and CD8 lymphocytes mediate suppression of hepatocellular carcinoma growth via tumor antigen-pulsed dendritic cells.Int J Cancer. 2003 Aug 20;106(2):236-43. doi: 10.1002/ijc.11201. Int J Cancer. 2003. PMID: 12800200
-
Induction of effective therapeutic antitumor immunity by direct in vivo administration of lentiviral vectors.Gene Ther. 2006 Apr;13(7):630-40. doi: 10.1038/sj.gt.3302697. Gene Ther. 2006. PMID: 16355115
-
Third generation dendritic cell vaccines for tumor immunotherapy.Eur J Cell Biol. 2012 Jan;91(1):53-8. doi: 10.1016/j.ejcb.2011.01.012. Epub 2011 Mar 25. Eur J Cell Biol. 2012. PMID: 21439674 Review.
-
T cells as vehicles for cancer vaccination.J Biomed Biotechnol. 2011;2011:417403. doi: 10.1155/2011/417403. Epub 2011 Oct 27. J Biomed Biotechnol. 2011. PMID: 22131805 Free PMC article. Review.
Cited by
-
Prevention and treatment of COVID-19: Focus on interferons, chloroquine/hydroxychloroquine, azithromycin, and vaccine.Biomed Pharmacother. 2021 Jan;133:111008. doi: 10.1016/j.biopha.2020.111008. Epub 2020 Nov 11. Biomed Pharmacother. 2021. PMID: 33227708 Free PMC article. Review.
-
Identification of new HLA-A*0201-restricted cytotoxic T lymphocyte epitopes from neuritin.J Neurooncol. 2013 Aug;114(1):51-8. doi: 10.1007/s11060-013-1167-6. Epub 2013 Jun 11. J Neurooncol. 2013. PMID: 23754640 Free PMC article.
-
Cancer Vaccines and Oncolytic Viruses Exert Profoundly Lower Side Effects in Cancer Patients than Other Systemic Therapies: A Comparative Analysis.Biomedicines. 2020 Mar 16;8(3):61. doi: 10.3390/biomedicines8030061. Biomedicines. 2020. PMID: 32188078 Free PMC article. Review.
-
Combined modality immunotherapy and chemotherapy: a new perspective.Cancer Immunol Immunother. 2008 Oct;57(10):1523-9. doi: 10.1007/s00262-008-0531-4. Epub 2008 May 17. Cancer Immunol Immunother. 2008. PMID: 18488219 Free PMC article. Review.
-
Diverse T-cell differentiation potentials of human fetal thymus, fetal liver, cord blood and adult bone marrow CD34 cells on lentiviral Delta-like-1-modified mouse stromal cells.Immunology. 2009 Sep;128(1 Suppl):e497-505. doi: 10.1111/j.1365-2567.2008.03013.x. Epub 2008 Nov 20. Immunology. 2009. PMID: 19740310 Free PMC article.
References
-
- Esche C, Shurin MR, Lotze MT. The use of dendritic cells for cancer vaccination. Curr Opin Mol Ther. 1999;1(1):72–81. - PubMed
-
- Kirk CJ, Mule JJ. Gene-modified dendritic cells for use in tumor vaccines. Hum Gene Ther. 2000;11(6):797–806. - PubMed
-
- Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15(2):138–47. - PubMed
-
- Ridgway D. The first 1000 dendritic cell vaccinees. Cancer Invest. 2003;21(6):873–86. - PubMed
-
- Banchereau J, Palucka AK. Dendritic cells as therapeutic vaccines against cancer. Nat Rev Immunol. 2005;5(4):296–306. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

