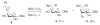On the regioselectivity of the Hanessian-Hullar reaction in 4,6-O-benzylidene protected galactopyranosides
- PMID: 16530738
- PMCID: PMC1559505
- DOI: 10.1016/j.carres.2006.02.024
On the regioselectivity of the Hanessian-Hullar reaction in 4,6-O-benzylidene protected galactopyranosides
Abstract
The N-bromosuccinimide mediated fragmentation of methyl 4,6-O-benzylidene-beta-D-galactopyranoside results in the formation of methyl 4-O-benzoyl-6-bromo-6-deoxy-beta-D-galactopyranoside and methyl 4-O-benzoyl-3-bromo-3-deoxy-beta-D-gulopyranoside, as opposed to the methyl 6-O-benzoyl-3-bromo-3-deoxy-beta-D-gulopyranoside originally reported. The kinetic methyl 4-O-benzoyl-6-bromo-6-deoxy-beta-D-galactopyranoside rearranges to the thermodynamic methyl 4-O-benzoyl-3-bromo-3-deoxy-beta-D-gulopyranoside under the reaction conditions, likely via a 3,6-anhydro galactopyranoside. The NBS-mediated cleavage of 4,6-O-benzylidene acetals in the galactopyranoside series is therefore shown to conform to the regiochemistry observed in the corresponding gluco- and mannopyranoside series with preferential cleavage of the C6-O6 bond by an ionic mechanism.
Figures
References
-
- Hanessian S. Carbohydr Res. 1966;2:86–88.
-
- Failla DL, Hullar TL, Siskin SB. J Chem Soc, Chem Commun. 1966:716–717.
-
- Hanessian S, Plessas NR. J Org Chem. 1969;34:1035–1044.
-
- Hanessian S, Plessas NR. J Org Chem. 1969;34:1045–1053.
-
- Hullar TL, Siskin SB. J Org Chem. 1970;35:225–227. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous