An improved grafting technique for mature Arabidopsis plants demonstrates long-distance shoot-to-root transport of phytochelatins in Arabidopsis
- PMID: 16531489
- PMCID: PMC1459314
- DOI: 10.1104/pp.105.072637
An improved grafting technique for mature Arabidopsis plants demonstrates long-distance shoot-to-root transport of phytochelatins in Arabidopsis
Abstract
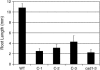
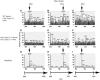
Phytochelatins (PCs) are peptides that function in heavy-metal chelation and detoxification in plants and fungi. A recent study showed that PCs have the ability to undergo long-distance transport in a root-to-shoot direction in transgenic Arabidopsis (Arabidopsis thaliana). To determine whether long-distance transport of PCs can occur in the opposite direction, from shoots to roots, the wheat (Triticum aestivum) PC synthase (TaPCS1) gene was expressed under the control of a shoot-specific promoter (CAB2) in an Arabidopsis PC-deficient mutant, cad1-3 (CAB2TaPCS1/cad1-3). Analyses demonstrated that TaPCS1 is expressed only in shoots and that CAB2TaPCS1/cad1-3 lines complement the cadmium (Cd) and arsenic metal sensitivity of cad1-3 shoots. CAB2TaPCS1/cad1-3 plants exhibited higher Cd accumulation in roots and lower Cd accumulation in shoots compared to wild type. Fluorescence HPLC coupled to mass spectrometry analyses directly detected PC2 in the roots of CAB2:TaPCS1/cad1-3 but not in cad1-3 controls, suggesting that PC2 is transported over long distances in the shoot-to-root direction. In addition, wild-type shoot tissues were grafted onto PC synthase cad1-3 atpcs2-1 double loss-of-function mutant root tissues. An Arabidopsis grafting technique for mature plants was modified to obtain an 84% success rate, significantly greater than a previous rate of approximately 11%. Fluorescence HPLC-mass spectrometry showed the presence of PC2, PC3, and PC4 in the root tissue of grafts between wild-type shoots and cad1-3 atpcs2-1 double-mutant roots, demonstrating that PCs are transported over long distances from shoots to roots in Arabidopsis.
Figures



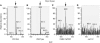

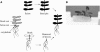

Similar articles
-
Long-distance root-to-shoot transport of phytochelatins and cadmium in Arabidopsis.Proc Natl Acad Sci U S A. 2003 Aug 19;100(17):10118-23. doi: 10.1073/pnas.1734072100. Epub 2003 Aug 8. Proc Natl Acad Sci U S A. 2003. PMID: 12909714 Free PMC article.
-
An HPLC-ICP-MS technique for determination of cadmium-phytochelatins in genetically modified Arabidopsis thaliana.J Chromatogr B Analyt Technol Biomed Life Sci. 2008 Jan 1;861(1):123-9. doi: 10.1016/j.jchromb.2007.11.004. Epub 2007 Nov 17. J Chromatogr B Analyt Technol Biomed Life Sci. 2008. PMID: 18065298 Free PMC article.
-
Influence of prior Cd(2+) exposure on the uptake of Cd(2+) and other elements in the phytochelatin-deficient mutant, cad1-3, of Arabidopsis thaliana.J Exp Bot. 2002 Mar;53(368):447-53. doi: 10.1093/jexbot/53.368.447. J Exp Bot. 2002. PMID: 11847243
-
Phytochelatins and Cadmium Mitigation: Harnessing Genetic Avenues for Plant Functional Manipulation.Int J Mol Sci. 2025 May 16;26(10):4767. doi: 10.3390/ijms26104767. Int J Mol Sci. 2025. PMID: 40429908 Free PMC article. Review.
-
Long-distance transport, vacuolar sequestration, tolerance, and transcriptional responses induced by cadmium and arsenic.Curr Opin Plant Biol. 2011 Oct;14(5):554-62. doi: 10.1016/j.pbi.2011.07.004. Epub 2011 Aug 5. Curr Opin Plant Biol. 2011. PMID: 21820943 Free PMC article. Review.
Cited by
-
Expression of Ceratophyllum demersum phytochelatin synthase, CdPCS1, in Escherichia coli and Arabidopsis enhances heavy metal(loid)s accumulation.Protoplasma. 2013 Dec;250(6):1263-72. doi: 10.1007/s00709-013-0508-9. Epub 2013 May 24. Protoplasma. 2013. PMID: 23702817
-
Identification of high levels of phytochelatins, glutathione and cadmium in the phloem sap of Brassica napus. A role for thiol-peptides in the long-distance transport of cadmium and the effect of cadmium on iron translocation.Plant J. 2008 Apr;54(2):249-59. doi: 10.1111/j.1365-313X.2008.03410.x. Epub 2008 Jan 16. Plant J. 2008. PMID: 18208526 Free PMC article.
-
Complexation of arsenite with phytochelatins reduces arsenite efflux and translocation from roots to shoots in Arabidopsis.Plant Physiol. 2010 Apr;152(4):2211-21. doi: 10.1104/pp.109.150862. Epub 2010 Feb 3. Plant Physiol. 2010. PMID: 20130102 Free PMC article.
-
Inflorescence stem grafting made easy in Arabidopsis.Plant Methods. 2012 Dec 19;8(1):50. doi: 10.1186/1746-4811-8-50. Plant Methods. 2012. PMID: 23249585 Free PMC article.
-
A pin-fasten grafting method provides a non-sterile and highly efficient method for grafting Arabidopsis at diverse developmental stages.Plant Methods. 2015 Jul 8;11:38. doi: 10.1186/s13007-015-0081-7. eCollection 2015. Plant Methods. 2015. PMID: 26157472 Free PMC article.
References
-
- Arteca RN, Arteca JM (2000) A novel method for growing Arabidopsis thaliana plants hydroponically. Physiol Plant 108: 188–193
-
- Cazalé A, Clemens S (2001) Arabidopsis thaliana expresses a second functional phytochelatin synthase. FEBS Lett 507: 215–219 - PubMed
-
- Chen A (2005) Long distance transport of phytochelatins in Arabidopsis and the isolation and characterization of cadmium tolerant mutants in Arabidopsis. PhD thesis. University of California, San Diego, pp 35–59
-
- Clarkson DT, Lüttge U (1989) Mineral nutrition. Divalent cations, transport and compartmentalization. Prog Bot 51: 93–112
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

