A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria
- PMID: 16531492
- PMCID: PMC1425857
- DOI: 10.1105/tpc.105.040121
A soluble carotenoid protein involved in phycobilisome-related energy dissipation in cyanobacteria
Abstract
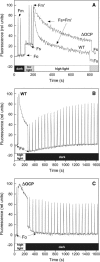
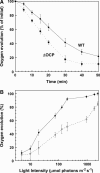
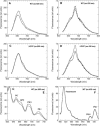
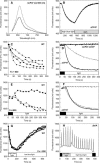
Photosynthetic organisms have developed multiple protective mechanisms to survive under high-light conditions. In plants, one of these mechanisms is the thermal dissipation of excitation energy in the membrane-bound chlorophyll antenna of photosystem II. The question of whether or not cyanobacteria, the progenitor of the chloroplast, have an equivalent photoprotective mechanism has long been unanswered. Recently, however, evidence was presented for the possible existence of a mechanism dissipating excess absorbed energy in the phycobilisome, the extramembrane antenna of cyanobacteria. Here, we demonstrate that this photoprotective mechanism, characterized by blue light-induced fluorescence quenching, is indeed phycobilisome-related and that a soluble carotenoid binding protein, ORANGE CAROTENOID PROTEIN (OCP), encoded by the slr1963 gene in Synechocystis PCC 6803, plays an essential role in this process. Blue light is unable to quench fluorescence in the absence of phycobilisomes or OCP. The fluorescence quenching is not DeltapH-dependent, and it can be induced in the absence of the reaction center II or the chlorophyll antenna, CP43 and CP47. Our data suggest that OCP, which strongly interacts with the thylakoids, acts as both the photoreceptor and the mediator of the reduction of the amount of energy transferred from the phycobilisomes to the photosystems. These are novel roles for a soluble carotenoid protein.
Figures




References
-
- Adir, N. (2005). Elucidation of the molecular structures of components of the phycobilisome: Reconstructing a giant. Photosynth. Res. 85 15–32. - PubMed
-
- Ajlani, G., and Vernotte, C. (1998). Construction and characterization of a phycobiliprotein-less mutant of Synechocystis sp. PCC 6803. Plant Mol. Biol. 37 577–580. - PubMed
-
- Ajlani, G., Vernotte, C., DiMagno, L., and Haselkorn, R. (1995). Phycobilisome core mutants of Synechocystis PCC 6803. Biochim. Biophys. Acta 1231 189–196.
-
- Allen, J.F. (1992). Protein phosphorylation in regulation of photosynthesis. Biochim. Biophys. Acta 1098 275–335. - PubMed
-
- Aro, E.M., Virgin, I., and Andersson, B. (1993). Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1143 113–134. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

