Calcium-dependent protein kinase isoforms in Petunia have distinct functions in pollen tube growth, including regulating polarity
- PMID: 16531501
- PMCID: PMC1425858
- DOI: 10.1105/tpc.105.037135
Calcium-dependent protein kinase isoforms in Petunia have distinct functions in pollen tube growth, including regulating polarity
Abstract
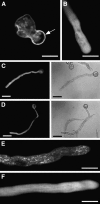
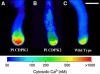
Calcium is a key regulator of pollen tube growth, but little is known concerning the downstream components of the signaling pathways involved. We identified two pollen-expressed calmodulin-like domain protein kinases from Petunia inflata, CALMODULIN-LIKE DOMAIN PROTEIN KINASE1 (Pi CDPK1) and Pi CDPK2. Transient overexpression or expression of catalytically modified Pi CDPK1 disrupted pollen tube growth polarity, whereas expression of Pi CDPK2 constructs inhibited tube growth but not polarity. Pi CDPK1 exhibited plasma membrane localization most likely mediated by acylation, and we present evidence that suggests this localization is critical to the biological function of this kinase. Pi CDPK2 substantially localized to as yet unidentified internal membrane compartments, and this localization was again, at least partially, mediated by acylation. In contrast with Pi CDPK1, altering the localization of Pi CDPK2 did not noticeably alter the effect of overexpressing this isoform on pollen tube growth. Ca(2+) requirements for Pi CDPK1 activation correlated closely with Ca(2+) concentrations measured in the growth zone at the pollen tube apex. Interestingly, loss of polarity associated with overexpression of Pi CDPK1 was associated with elevated cytosolic Ca(2+) throughout the bulging tube tip, suggesting that Pi CDPK1 may participate in maintaining Ca(2+) homeostasis. These results are discussed in relation to previous models for Ca(2+) regulation of pollen tube growth.
Figures


References
-
- Allwood, E.G., Smertenko, A.P., and Hussey, P.J. (2001). Phosphorylation of plant actin-depolymerizing factor by calmodulin-like domain protein kinase. FEBS Lett. 499 97–100. - PubMed
-
- Bamburg, J.R. (1999). Proteins of the ADF/cofilin family: Essential regulators of actin dynamics. Annu. Rev. Cell Biol. 15 185–230. - PubMed
-
- Battey, N.H., and Blackbourne, H.D. (1993). The control of exocytosis in plant cells. New Phytol. 125 307–338. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

