Anti-EphA2 antibodies decrease EphA2 protein levels in murine CT26 colorectal and human MDA-231 breast tumors but do not inhibit tumor growth
- PMID: 16533422
- PMCID: PMC1584286
- DOI: 10.1593/neo.05544
Anti-EphA2 antibodies decrease EphA2 protein levels in murine CT26 colorectal and human MDA-231 breast tumors but do not inhibit tumor growth
Abstract
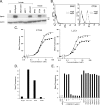
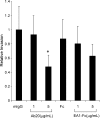
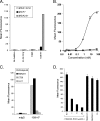
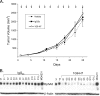
The EphA2 receptor tyrosine kinase has been shown to be over-expressed in cancer and a monoclonal antibody (mAb) that activates and down-modulates EphA2 was reported to inhibit the growth of human breast and lung tumor xenografts in nude mice. Reduction of EphA2 levels by treatment with anti-EphA2 siRNA also inhibited tumor growth, suggesting that the anti-tumor effects of these agents are mediated by decreasing the levels of EphA2. As these studies employed human tumor xenograft models in nude mice with reagents whose cross reactivity with murine EphA2 is unknown, we generated a mAb (Ab20) that preferentially binds, activates, and induces the degradation of murine EphA2. Treatment of established murine CT26 colorectal tumors with Ab20 reduced EphA2 protein levels to approximately 12% of control tumor levels, yet had no effect on tumor growth. CT26 tumor cell colonization of the lung was also not affected by Ab20 administration despite having barely detectable levels of EphA2. We also generated and tested a potent agonistic mAb against human EphA2 (1G9-H7). No inhibition of humanMDA-231 breast tumor xenograft growth was observed despite evidence for >85% reduction of EphA2 protein levels in the tumors. These results suggest that molecular characteristics of the tumors in addition to EphA2 over-expression may be important for predicting responsiveness to EphA2-directed therapies.
Figures




Similar articles
-
Antibody-dependent cell-mediated cytotoxicity effector-enhanced EphA2 agonist monoclonal antibody demonstrates potent activity against human tumors.Neoplasia. 2009 Jun;11(6):509-17, 2 p following 517. doi: 10.1593/neo.81578. Neoplasia. 2009. PMID: 19484140 Free PMC article.
-
EphrinA1-EphA2 interaction-mediated apoptosis and FMS-like tyrosine kinase 3 receptor ligand-induced immunotherapy inhibit tumor growth in a breast cancer mouse model.J Gene Med. 2012 Feb;14(2):77-89. doi: 10.1002/jgm.1649. J Gene Med. 2012. PMID: 22228563 Free PMC article.
-
Novel anti-EPHA2 antibody, DS-8895a for cancer treatment.Cancer Biol Ther. 2016 Nov;17(11):1158-1167. doi: 10.1080/15384047.2016.1235663. Epub 2016 Sep 21. Cancer Biol Ther. 2016. PMID: 27653549 Free PMC article.
-
EphA2 overexpression decreases estrogen dependence and tamoxifen sensitivity.Cancer Res. 2003 Jun 15;63(12):3425-9. Cancer Res. 2003. PMID: 12810680
-
EphA2-dependent molecular targeting therapy for malignant tumors.Curr Cancer Drug Targets. 2011 Nov;11(9):1082-97. doi: 10.2174/156800911798073050. Curr Cancer Drug Targets. 2011. PMID: 21933105 Review.
Cited by
-
Antibody Targeting of Eph Receptors in Cancer.Pharmaceuticals (Basel). 2020 May 8;13(5):88. doi: 10.3390/ph13050088. Pharmaceuticals (Basel). 2020. PMID: 32397088 Free PMC article. Review.
-
Eph receptors and ephrins: therapeutic opportunities.Annu Rev Pharmacol Toxicol. 2015;55:465-87. doi: 10.1146/annurev-pharmtox-011112-140226. Epub 2014 Oct 3. Annu Rev Pharmacol Toxicol. 2015. PMID: 25292427 Free PMC article. Review.
-
Antibody-dependent cell-mediated cytotoxicity effector-enhanced EphA2 agonist monoclonal antibody demonstrates potent activity against human tumors.Neoplasia. 2009 Jun;11(6):509-17, 2 p following 517. doi: 10.1593/neo.81578. Neoplasia. 2009. PMID: 19484140 Free PMC article.
-
The gene polymorphism of the angiotensin I-converting enzyme correlates with tumor size and patient survival in colorectal cancer patients.Neoplasia. 2007 Sep;9(9):716-22. doi: 10.1593/neo.07418. Neoplasia. 2007. PMID: 17898867 Free PMC article.
-
The SAM domains of Anks family proteins are critically involved in modulating the degradation of EphA receptors.Mol Cell Biol. 2010 Apr;30(7):1582-92. doi: 10.1128/MCB.01605-09. Epub 2010 Jan 25. Mol Cell Biol. 2010. PMID: 20100865 Free PMC article.
References
-
- Kullander K, Klein R. Mechanisms and functions of Eph and ephrin signalling. Nat Rev Mol Cell Biol. 2002;3:475–486. - PubMed
-
- Surawska H, Ma PC, Salgia R. The role of ephrins and Eph receptors in cancer. Cytokine Growth Factor Rev. 2004;15:419–433. - PubMed
-
- Zelinski DP, Zantek ND, Stewart JC, Irizarry AR, Kinch MS. EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 2001;61:2301–2306. - PubMed
-
- Walker-Daniels J, Coffman K, Azimi M, Rhim JS, Bostwick DG, Snyder P, Kerns BJ, Waters DJ, Kinch MS. Overexpression of the EphA2 tyrosine kinase in prostate cancer. Prostate. 1999;41:275–280. - PubMed
-
- Thaker PH, Deavers M, Celestino J, Thornton A, Fletcher MS, Landen CN, Kinch MS, Kiener PA, Sood AK. EphA2 expression is associated with aggressive features in ovarian carcinoma. Clin Cancer Res. 2004;10:5145–5150. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
