Expression studies in gliomas and glial cells do not support a tumor suppressor role for LGI1
- PMID: 16533756
- PMCID: PMC1871933
- DOI: 10.1215/15228517-2005-006
Expression studies in gliomas and glial cells do not support a tumor suppressor role for LGI1
Abstract
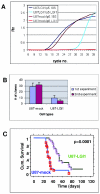
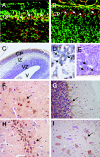
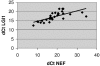
Disruptions of LGI1 in glioblastoma (GBM) cell lines and LGI1 mutations in families with autosomal dominant epilepsy imply a role for LGI1 in glial cells as well as in neurons. Although we and others could not find LGI1 mutations in malignant gliomas, our initial studies appeared to support the idea that LGI1 is poorly expressed or absent in these tumors. Microarray data suggested that LGI1 could be involved in the control of matrix metalloproteinases, and we found that tumors derived from U87 glioblastoma cells overexpressing LGI1 were less aggressive than U87 control tumors. To our surprise, we observed that LGI1 expression after differentiation of murine neural stem cells was robust in neurons but negligible in glial cells, in agreement with immunohistochemistry studies on rodent brain. This observation could suggest that the variable levels of LGI1 expression in gliomas reflect the presence of neurons entrapped within the tumor. To test this hypothesis, we investigated LGI1 expression in parallel with expression of the neuronal marker NEF3 by real-time PCR on 30 malignant gliomas. Results showed a strong, positive correlation between the expression levels of these two genes (P < 0.0001). Thus, our data confirm that LGI1 is involved in cell-matrix interactions but suggest that its expression is not relevant in glial cells, implying that its role as a tumor suppressor in gliomas should be reconsidered.
Figures




References
-
- Albini A, Benelli R, Noonan DM, Brigati C. The “chemoin-vasion assay”: A tool to study tumor and endothelial cell invasion of basement membranes. Int J Dev Biol. 2004;48:563–571. - PubMed
-
- Apte SS, Olsen BR, Murphy G. The gene structure of tissue inhibitor of metalloproteinases (TIMP)-3 and its inhibitory activities define the distinct TIMP gene family (erratum in J. Biol. Chem. [1996] 271, 2874) J Biol Chem. 1995;270:14313–14318. - PubMed
-
- Arato-Ohshima T, Sawa H. Over-expression of cyclin D1 induces glioma invasion by increasing matrix metalloproteinase activity and cell motility. Int J Cancer. 1999;83:387–392. - PubMed
-
- Bachman KE, Herman JG, Corn PG, Merlo A, Costello JF, Cavenee WK, Baylin SB, Graff JR. Methylation-associated silencing of the tissue inhibitor of metalloproteinase-3 gene suggests a suppressor role in kidney, brain, and other human cancers. Cancer Res. 1999;59:798–802. - PubMed

