Kinetics of metastatic breast cancer cell trafficking in bone
- PMID: 16533765
- PMCID: PMC1523260
- DOI: 10.1158/1078-0432.CCR-05-1806
Kinetics of metastatic breast cancer cell trafficking in bone
Abstract
Purpose: In vivo studies have focused on the latter stages of the bone metastatic process (osteolysis), whereas little is known about earlier events, e.g., arrival, localization, and initial colonization. Defining these initial steps may potentially identify the critical points susceptible to therapeutic intervention.
Experimental design: MDA-MB-435 human breast cancer cells engineered with green fluorescent protein were injected into the cardiac left ventricle of athymic mice. Femurs were analyzed by fluorescence microscopy, immunohistochemistry, real-time PCR, flow cytometry, and histomorphometry at times ranging from 1 hour to 6 weeks.
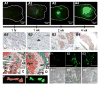
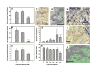
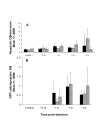
Results: Single cells were found in distal metaphyses at 1 hour postinjection and remained as single cells up to 72 hours. Diaphyseal arrest occurred rarely and few cells remained there after 24 hours. At 1 week, numerous foci (2-10 cells) were observed, mostly adjacent to osteoblast-like cells. By 2 weeks, fewer but larger foci (> or =50 cells) were seen. Most bones had a single large mass at 4 weeks (originating from a colony or coalescing foci) which extended into the diaphysis by 4 to 6 weeks. Little change (<20%) in osteoblast or osteoclast numbers was observed at 2 weeks, but at 4 to 6 weeks, osteoblasts were dramatically reduced (8% of control), whereas osteoclasts were reduced modestly (to approximately 60% of control).
Conclusions: Early arrest in metaphysis and minimal retention in diaphysis highlight the importance of the local milieu in determining metastatic potential. These results extend the Seed and Soil hypothesis by demonstrating both intertissue and intratissue differences governing metastatic location. Ours is the first in vivo evidence that tumor cells influence not only osteoclasts, as widely believed, but also eliminate functional osteoblasts, thereby restructuring the bone microenvironment to favor osteolysis. The data may also explain why patients receiving bisphosphonates fail to heal bone despite inhibiting resorption, implying that concurrent strategies that restore osteoblast function are needed to effectively treat osteolytic bone metastases.
Figures


References
-
- Body JJ. Metastatic bone disease: clinical and therapeutic aspects. Bone. 1992;13 (Suppl 1):S57–S62. - PubMed
-
- Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80:1588–94. - PubMed
-
- Mundy GR. Metastasis: Metastasis to bone: causes, consequences and therapeutic opportunities. Nature Rev Cancer. 2002;2:584–93. - PubMed
-
- Lelekakis M, Moseley JM, Martin TJ, et al. A novel orthotopic model of breast cancer metastasis to bone. Clin Exptl Metastasis. 1999;17:163–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

