Model of chromosome motility in Drosophila embryos: adaptation of a general mechanism for rapid mitosis
- PMID: 16533843
- PMCID: PMC1459506
- DOI: 10.1529/biophysj.105.078691
Model of chromosome motility in Drosophila embryos: adaptation of a general mechanism for rapid mitosis
Abstract
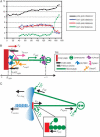
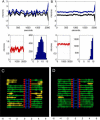
During mitosis, ensembles of dynamic MTs and motors exert forces that coordinate chromosome segregation. Typically, chromosomes align at the metaphase spindle equator where they oscillate along the pole-pole axis before disjoining and moving poleward during anaphase A, but spindles in different cell types display differences in MT dynamicity, in the amplitude of chromosome oscillations and in rates of chromatid-to-pole motion. Drosophila embryonic mitotic spindles, for example, display remarkably dynamic MTs, barely detectable metaphase chromosome oscillations, and a rapid rate of "flux-pacman-dependent" anaphase chromatid-to-pole motility. Here we develop a force-balance model that describes Drosophila embryo chromosome motility in terms of a balance of forces acting on kinetochores and kMTs that is generated by multiple polymer ratchets and mitotic motors coupled to tension-dependent kMT dynamics. The model shows that i), multiple MTs displaying high dynamic instability can drive steady and rapid chromosome motion; ii), chromosome motility during metaphase and anaphase A can be described by a single mechanism; iii), high kinetochore dynein activity is deployed to dampen metaphase oscillations, to augment the basic flux-pacman mechanism, and to drive rapid anaphase A; iv), modulation of the MT rescue frequency by the kinetochore-associated kinesin-13 depolymerase promotes metaphase chromosome oscillations; and v), this basic mechanism can be adapted to a broad range of spindles.
Figures


References
-
- McIntosh, J. R., E. L. Grishchuk, and R. R. West. 2002. Chromosome-microtubule interactions during mitosis. Annu. Rev. Cell Dev. Biol. 18:193–219. - PubMed
-
- Maiato, H., and C. E. Sunkel. 2004. Kinetochore-microtubule interactions during cell division. Chromosome Res. 12:585–597. - PubMed
-
- Kwon, M., and J. M. Scholey. 2004. Spindle mechanics and dynamics during mitosis in Drosophila. Trends Cell Biol. 14:194–205. - PubMed
-
- Rieder, C. L. 1982. The formation, structure, and composition of the mammalian kinetochore and kinetochore fiber. Int. Rev. Cytol. 79:1–58. - PubMed
-
- Maiato, H., J. DeLuca, E. D. Salmon, and W. C. Earnshaw. 2004. The dynamic kinetochore-microtubule interface. J. Cell Sci. 117:5461–5477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

