Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling
- PMID: 16533949
- PMCID: PMC2063734
- DOI: 10.1083/jcb.200508130
Osteoblast differentiation and skeletal development are regulated by Mdm2-p53 signaling
Abstract
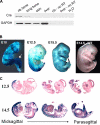
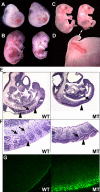
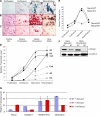
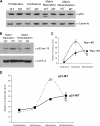
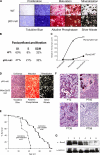
Mdm2 is required to negatively regulate p53 activity at the peri-implantation stage of early mouse development. However, the absolute requirement for Mdm2 throughout embryogenesis and in organogenesis is unknown. To explore Mdm2-p53 signaling in osteogenesis, Mdm2-conditional mice were bred with Col3.6-Cre-transgenic mice that express Cre recombinase in osteoblast lineage cells. Mdm2-conditional Col3.6-Cre mice die at birth and display multiple skeletal defects. Osteoblast progenitor cells deleted for Mdm2 have elevated p53 activity, reduced proliferation, reduced levels of the master osteoblast transcriptional regulator Runx2, and reduced differentiation. In contrast, p53-null osteoprogenitor cells have increased proliferation, increased expression of Runx2, increased osteoblast maturation, and increased tumorigenic potential, as mice specifically deleted for p53 in osteoblasts develop osteosarcomas. These results demonstrate that p53 plays a critical role in bone organogenesis and homeostasis by negatively regulating bone development and growth and by suppressing bone neoplasia and that Mdm2-mediated inhibition of p53 function is a prerequisite for Runx2 activation, osteoblast differentiation, and proper skeletal formation.
Figures


Comment in
-
Skeletons in the p53 tumor suppressor closet: genetic evidence that p53 blocks bone differentiation and development.J Cell Biol. 2006 Mar 13;172(6):795-7. doi: 10.1083/jcb.200601114. J Cell Biol. 2006. PMID: 16533941 Free PMC article. Review.
References
-
- Armstrong, J.F., M.H. Kaufman, D.J. Harrison, and A.R. Clarke. 1995. High-frequency developmental abnormalities in p53-deficient mice. Curr. Biol. 5:931–936. - PubMed
-
- Banerjee, C., L.R. McCabe, J.-Y. Choi, S.W. Hiebert, J.L. Stein, G.S. Stein, and J.B. Lian. 1997. Runt homology domain proteins in osteoblast differentiation: AML3/CBFA1 is a major component of a bone-specific complex. J. Cell. Biochem. 66:1–8. - PubMed
-
- Deng, C., P. Zhang, J.W. Harper, S.J. Elledge, and P. Leder. 1995. Mice lacking p21CIP1/WAF1 undergo normal development, but are defective in G1 checkpoint control. Cell. 82:675–684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

